Hello this is my first time building water from RO and I have used ez water calculator but I am a bit hesitant as to whether I did it correctly or not.
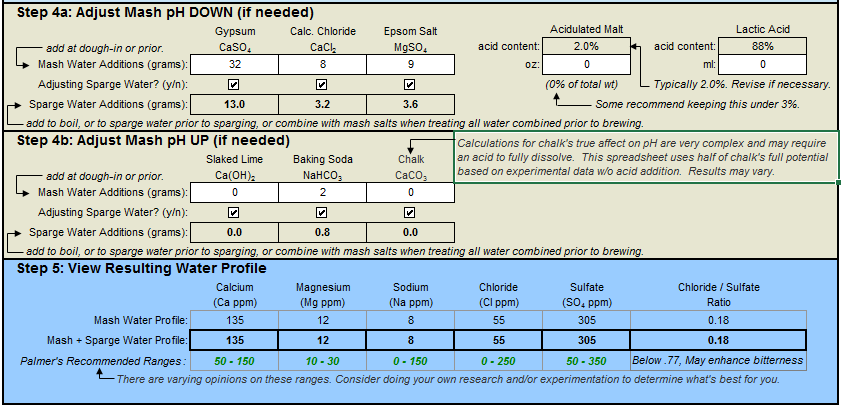
I used another thread in hbt where a guy showed the target water profile of:
Ca+2 -- 140
Mg+2 -- 12
Na+ --7
CL- -- 55
SO4-2 -- 300
HCO3- -- 0
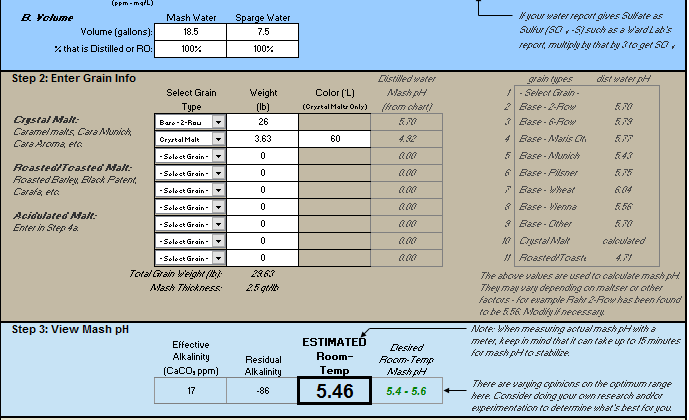
Total water for brew day including sparge = 100L
Base 2-Row = 26lb
Crystal (60L) = 3.63LB
Using the EZ Water Adjustment, I got these additions.
35g Gypsum
11.2 Calc. Chloride
12.6g Epsom Salt
2.8g Baking Soda
Everything on the "Resulting Water Profile" falls exactly within the range of the target water profile and is in the green. But I am not confident that I have done everything properly.
Do these additions look about right for the amount of water and malts I am using?
Thanks very much for the help!
I used another thread in hbt where a guy showed the target water profile of:
Ca+2 -- 140
Mg+2 -- 12
Na+ --7
CL- -- 55
SO4-2 -- 300
HCO3- -- 0
Total water for brew day including sparge = 100L
Base 2-Row = 26lb
Crystal (60L) = 3.63LB
Using the EZ Water Adjustment, I got these additions.
35g Gypsum
11.2 Calc. Chloride
12.6g Epsom Salt
2.8g Baking Soda
Everything on the "Resulting Water Profile" falls exactly within the range of the target water profile and is in the green. But I am not confident that I have done everything properly.
Do these additions look about right for the amount of water and malts I am using?
Thanks very much for the help!



Last edited:












































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)












