palobrewer
New Member
- Joined
- Oct 15, 2016
- Messages
- 4
- Reaction score
- 0
Hi,
I started kegging for the first time a couple days ago and I have a question regarding the loss of CO2. I'm not sure if I still have a leak or the Co2 is being absorbed into the gas.
Any comments or suggestions would be greatly appreciated.
Day 1
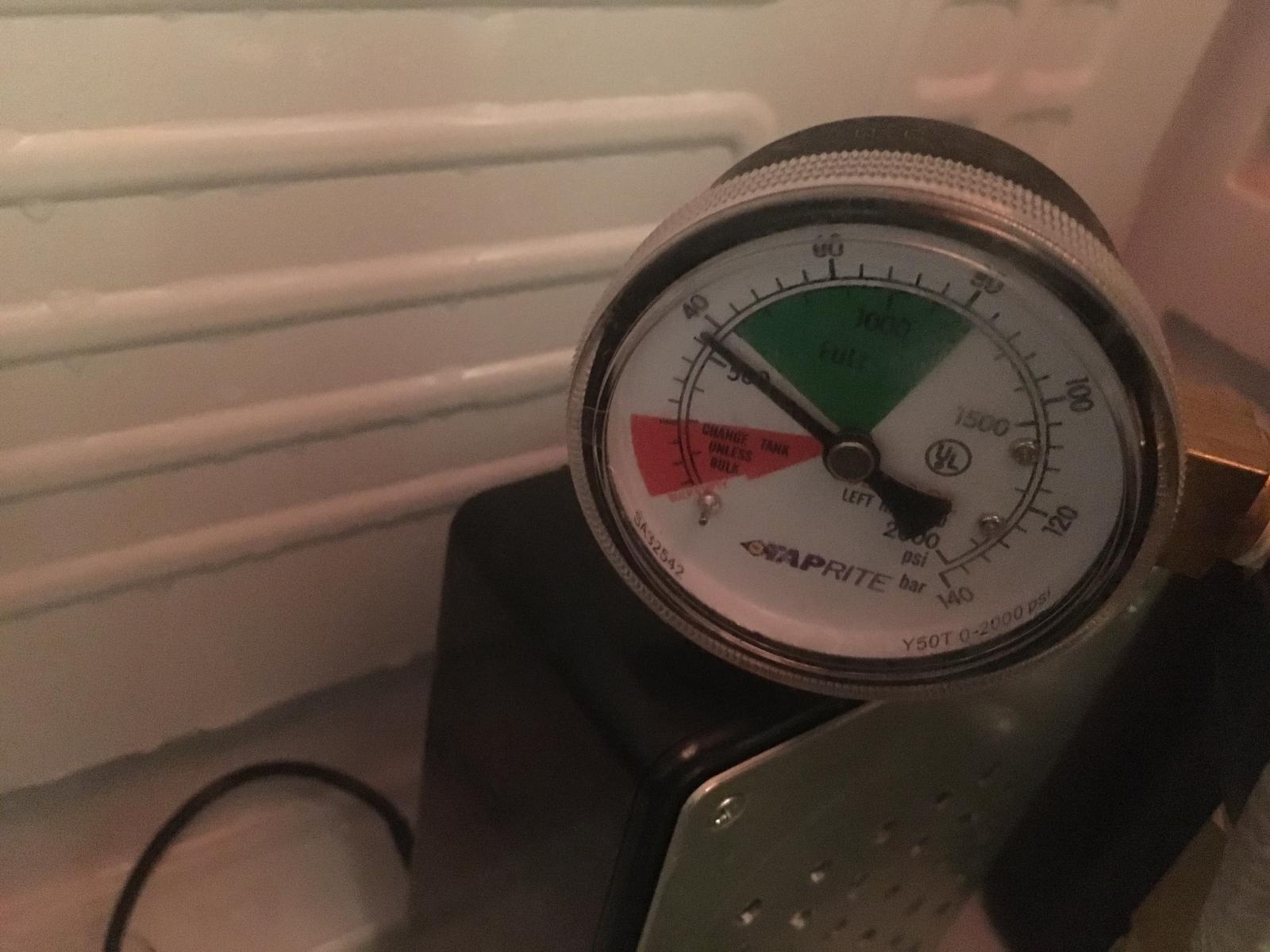
I wanted to force carb the keg in my mini fridge with 30 PSI for 24 hours, however, after 12 hours I check the regulator and it had lost 20 Bar of CO2. Started at 60 Bar then went down to 40 Bar of co2. So I believe this was a leak
Day 2
I took the Co2 out of the fridge and the gauge slowly went up to 48 Bar of Co2. I used the soap water test and found some joints that were bubbling and tightened everything again.
Day 3
I placed the Co2 tank back in the fridge and the following morning I read the gauge and it's around 40 Bar of co2.
Setup
- Mini-Fridge at 37 degrees F
- 3 Gallon Ball Lock Corny Keg
- 5 Lb Co2 Tank
- Taprite Dual Gauge MFL Regulator
- John Guest Fittings
- 3/8" Nylon Braided Tube


I started kegging for the first time a couple days ago and I have a question regarding the loss of CO2. I'm not sure if I still have a leak or the Co2 is being absorbed into the gas.
Any comments or suggestions would be greatly appreciated.
Day 1
I wanted to force carb the keg in my mini fridge with 30 PSI for 24 hours, however, after 12 hours I check the regulator and it had lost 20 Bar of CO2. Started at 60 Bar then went down to 40 Bar of co2. So I believe this was a leak
Day 2
I took the Co2 out of the fridge and the gauge slowly went up to 48 Bar of Co2. I used the soap water test and found some joints that were bubbling and tightened everything again.
Day 3
I placed the Co2 tank back in the fridge and the following morning I read the gauge and it's around 40 Bar of co2.
Setup
- Mini-Fridge at 37 degrees F
- 3 Gallon Ball Lock Corny Keg
- 5 Lb Co2 Tank
- Taprite Dual Gauge MFL Regulator
- John Guest Fittings
- 3/8" Nylon Braided Tube





