Spidermonkey
New Member
- Joined
- Apr 12, 2018
- Messages
- 2
- Reaction score
- 1
Hello everybody,
I'm new here so I hope I'm not making any violations.
I kind of need some advise.
So I finally set up my system last night.
It's a kegerator with double taps for some home brew.
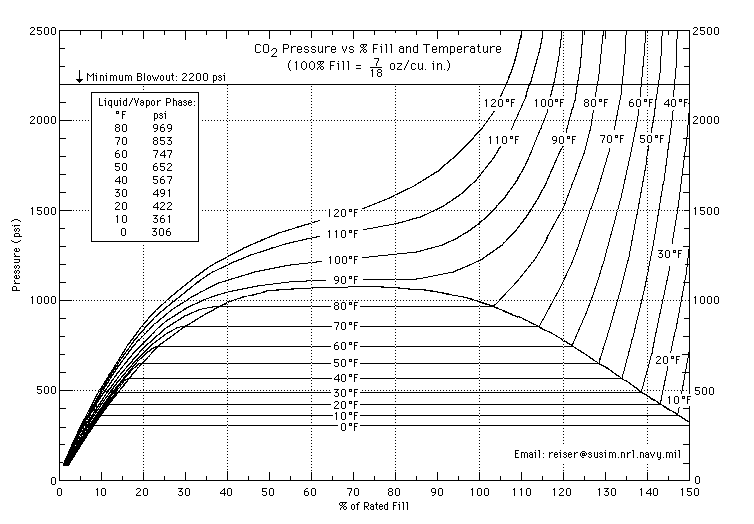
My question is last night after putting everything together and checking for leaks( there were none) my system was at 750 psi.
I have a 20 lb tank. The tank and fridge were warm when I set it up.
This morning when I woke up everything was nice and cold inside
HOWEVER... I noticed that my tank pressure dropped from 750 psi to 525psi...?????
I was like WTH....
I checked for leaks and re tightened all the clamps , still no leak....
Is this normal or something I should be concerned with?
Thank you again for your help.
Eric
I'm new here so I hope I'm not making any violations.
I kind of need some advise.
So I finally set up my system last night.
It's a kegerator with double taps for some home brew.
My question is last night after putting everything together and checking for leaks( there were none) my system was at 750 psi.
I have a 20 lb tank. The tank and fridge were warm when I set it up.
This morning when I woke up everything was nice and cold inside
HOWEVER... I noticed that my tank pressure dropped from 750 psi to 525psi...?????
I was like WTH....
I checked for leaks and re tightened all the clamps , still no leak....
Is this normal or something I should be concerned with?
Thank you again for your help.
Eric