I'm pretty sure your math is way off. at 30psi at 65°F on a tank you get 2.5vol equilibrium.
edit - sorry, read your post wrong; but that's maintaining 30 PSI rather than a single charge of 30 PSI, right?

I'm pretty sure your math is way off. at 30psi at 65°F on a tank you get 2.5vol equilibrium.
Bit of a different question, but could the "one shot" headspace pressure to carb a beer to equilibrium be calculated?
I don't mean like set it to 12psi (or whatever) and forget it for two weeks, I mean like put 5 gal of beer from fermenter into a corny keg then hit it with one charge of CO2 that would in time to bring it to 2.5vol from residual of 0.9 vol. What pressure would that take, in theory?
You can use the information in this post to do an accurate calculation.Depends on the headspace. I image it would be quite high

68F. Still not quite right though, since I didn't account for the CO2 left in the headspace at equilibrium.
oopsIn this hypothetical question the beer is at 34F
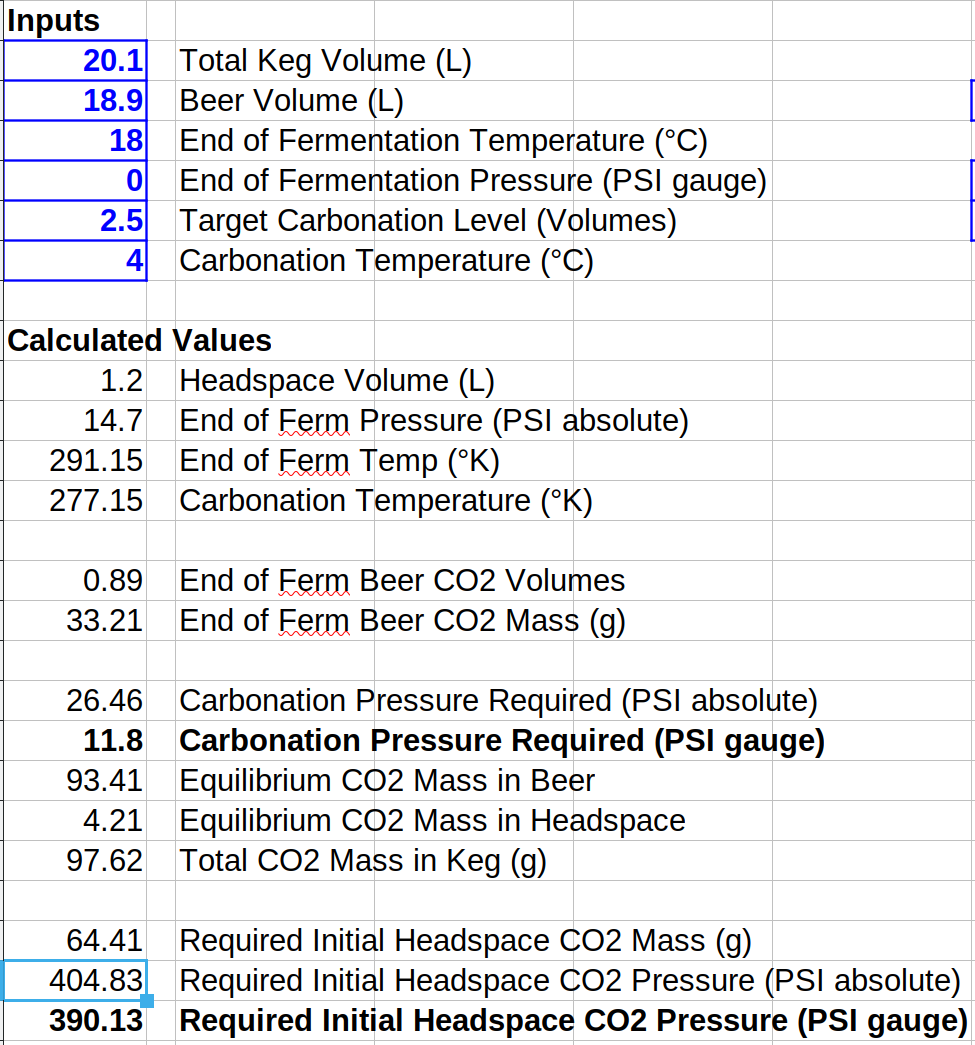
I put together a little spreadsheet to do this calculation. For18.9 L (5 gal) of beer fermented at atmospheric pressure and 18°C (64.4°F) to be carbonated to 2.5 volumes at 4°C (39.2°F.) The headspace volume is 1.2 L. The required single shot CO2 pressure for this case is 390 psig! Here's a screen shot of the spreadsheet, and the sheet is attached below (LibreOffice format only.)You can use the information in this post to do an accurate calculation.
Use the first part (the blue part) of the first equation to calculate the mass of CO2 in the beer at the end of fermentation. PA is the absolute fermentation pressure (14.7 psi for an open or airlocked fermentation, and 14.7 + gauge pressure for a pressure fermentation.) Then use the full first equation to calculate the mass of CO2 required in the keg. Here PA is 14.7 + gauge pressure for the desired level of carbonation. Now subtract the starting mass of CO2 in the beer from the total final mass of CO2 in the keg to give you how much CO2 you would have to force into the headspace. Now solve the second part of the first equation (the green part) for PA given the required headspace mass of CO2. Remember to subtract 14.7 from PA to get the gauge pressure.
For reference the total enclosed volume of a corny keg is 5.3 - 5.35 gal (20 - 20.25 L.)
Brew on



![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)











So if it really meant that much to someone, it looks like you could probably do a single shot pressurization / carbonation at a reasonable gauge pressure of ~60 PSI in a refrigerated corny keg if you reduced the beer volume to about 3.5 gallons.I put together a little spreadsheet to do this calculation.
I wonder how them Germans go about spunding stuff fully carbonated then. Is it because they are still krausening it as they slowly lower the temperatures so that there is a constant pressure source?The required single shot CO2 pressure for this case is 390 psig!
Or yeah use a lot more headspace than fluid.So if it really meant that much to someone, it looks like you could probably do a single shot pressurization / carbonation at a reasonable gauge pressure of ~60 PSI in a refrigerated corny keg if you reduced the beer volume to about 3.5 gallons.
I put together a little spreadsheet to do this calculation. For18.9 L (5 gal) of beer fermented at atmospheric pressure and 18°C (64.4°F) to be carbonated to 2.5 volumes at 4°C (39.2°F.) The headspace volume is 1.2 L. The required single shot CO2 pressure for this case is 390 psig! Here's a screen shot of the spreadsheet, and the sheet is attached below (LibreOffice format only.)
Brew on
When spunding, you have active fermentation going on, which creates CO2 in the beer. Then "excess" CO2 diffuses out of the beer to pressurize the headspace, and keep the headspace pressure in equilibrium with the carbonation level in the beer. As spunding continues, the carbonation level increases, as does the headspace pressure, but the headspace pressure is never above the equilibrium pressure (which is a requirement for forced carbonation.)I wonder how them Germans go about spunding stuff fully carbonated then. Is it because they are still krausening it as they slowly lower the temperatures so that there is a constant pressure source?

So they need to reach an equilibrium (or guess what it will be) before they cold crash from their krausening which will allow them to have the right amount of CO2 in headspace and dissolved to reach the right equilibrium at cold crash/lagering and bottling.When spunding, you have active fermentation going on, which creates CO2 in the beer. Then "excess" CO2 diffuses out of the beer to pressurize the headspace, and keep the headspace pressure in equilibrium with the carbonation level in the beer. As spunding continues, the carbonation level increases, as does the headspace pressure, but the headspace pressure is never above the equilibrium pressure (which is a requirement for forced carbonation.)
In the single shot forced carbonation case, there is no CO2 being produced within the beer, and all of the CO2 needed to carbonate the beer (less the residual CO2 from primary fermentation) must come from the headspace. If the headspace is small compared to the beer volume, then high pressures are required to get the requisite amount of CO2 in the headspace to start.
Brew on
No guessing needs to be involved. If you spund to the correct pressure, you will have the desired amount of carbonation in the beer. The spunding valve will release any excess CO2 from the headspace and beer. Cold crashing by itself doesn't change the amount of CO2 in the beer, but if you hold the cold crash for several days, you will absorb a little CO2 from the headspace. The larger the headspace to beer volume ratio, the more CO2 will be absorbed from the headspace when equilibrium is reached.So they need to reach an equilibrium (or guess what it will be) before they cold crash from their krausening which will allow them to have the right amount of CO2 in headspace and dissolved to reach the right equilibrium at cold crash/lagering and bottling.
I imagine that takes < 45 PSI


I did say cold crash/lagering. Lagering is going to be at equilibrium by the end so it will definitely change the amount of CO2. The general case is you do have to consider it, its only in the specific case of a short cold crash you don't.but if you hold the cold crash for several days, you will absorb a little CO2 from the headspace.
As far as lagering goes, it will depend on if you bulk lager in the fermenter and let the headspace pressure and CO2 pick-up do what they will. Or, if you transfer the beer to a bright tank or package the beer for lagering, where you can adjust the headspace CO2 partial pressure to a value that will leave you with what you want after lagering.I did say cold crash/lagering. Lagering is going to be at equilibrium by the end so it will definitely change the amount of CO2. The general case is you do have to consider it, its only in the specific case of a short cold crash you don't.
