aeviaanah
Well-Known Member
- Joined
- Jul 1, 2012
- Messages
- 1,686
- Reaction score
- 217
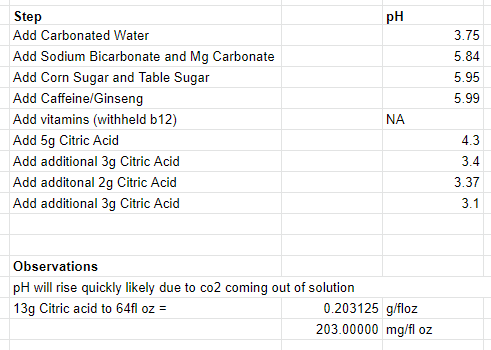
I am looking to reverse engineer Redbull energy drink and brew something similar. I am able to reference the back of can, nutritional facts and general online searches to solve for grams/oz of caffeine, taurine, vitamins etc. Where I am stuck on is pH and additions that affect pH such as the carbonated water, baking soda, magnesium and citric acid.
The wiki source below mentions Redbull prepares a buffer solution of carbonated water, baking soda and magnesium carbonate. When I google/youtube "buffer solution" there seems to be some science here I don't understand. I understand the finish pH needs to be around 3.3. I assume the baking soda and magnesium carbonate are base and will raise the pH.
Questions:
How to make a buffer solution using the ingredients listed above?
How much magnesium carbonate?
Can I use grams of sodium from back of can to solve for baking soda addition (by means of sodium contribution)?
Would I add carbonated water, baking soda and magnesium carbonate and then add the citric acid to pH of 3.3?
The wiki source is below
"Depending on the country, Red Bull contains different amounts of caffeine, taurine, B vitamins (B2, B3, B5, B6, B12) and simple sugars (sucrose and glucose) in a buffer solution of carbonated water, baking soda and magnesium carbonate (substituted in some flavours with a trisodium citrate/citric acid buffer, each solution providing electrolytes).[40][41]"
The wiki source below mentions Redbull prepares a buffer solution of carbonated water, baking soda and magnesium carbonate. When I google/youtube "buffer solution" there seems to be some science here I don't understand. I understand the finish pH needs to be around 3.3. I assume the baking soda and magnesium carbonate are base and will raise the pH.
Questions:
How to make a buffer solution using the ingredients listed above?
How much magnesium carbonate?
Can I use grams of sodium from back of can to solve for baking soda addition (by means of sodium contribution)?
Would I add carbonated water, baking soda and magnesium carbonate and then add the citric acid to pH of 3.3?
The wiki source is below
"Depending on the country, Red Bull contains different amounts of caffeine, taurine, B vitamins (B2, B3, B5, B6, B12) and simple sugars (sucrose and glucose) in a buffer solution of carbonated water, baking soda and magnesium carbonate (substituted in some flavours with a trisodium citrate/citric acid buffer, each solution providing electrolytes).[40][41]"












![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)