Now I must confessing there are waters described on this forum which are vastly different to any waters I ever experienced or read of in the UK where I live, but this is another of those.
There is an infinite number of ways in which ions can combine depending on the geological underpinnings of the water supply, local influences such as agricultural runoff and salt treatment of roads in winter and how the utility treats the water.
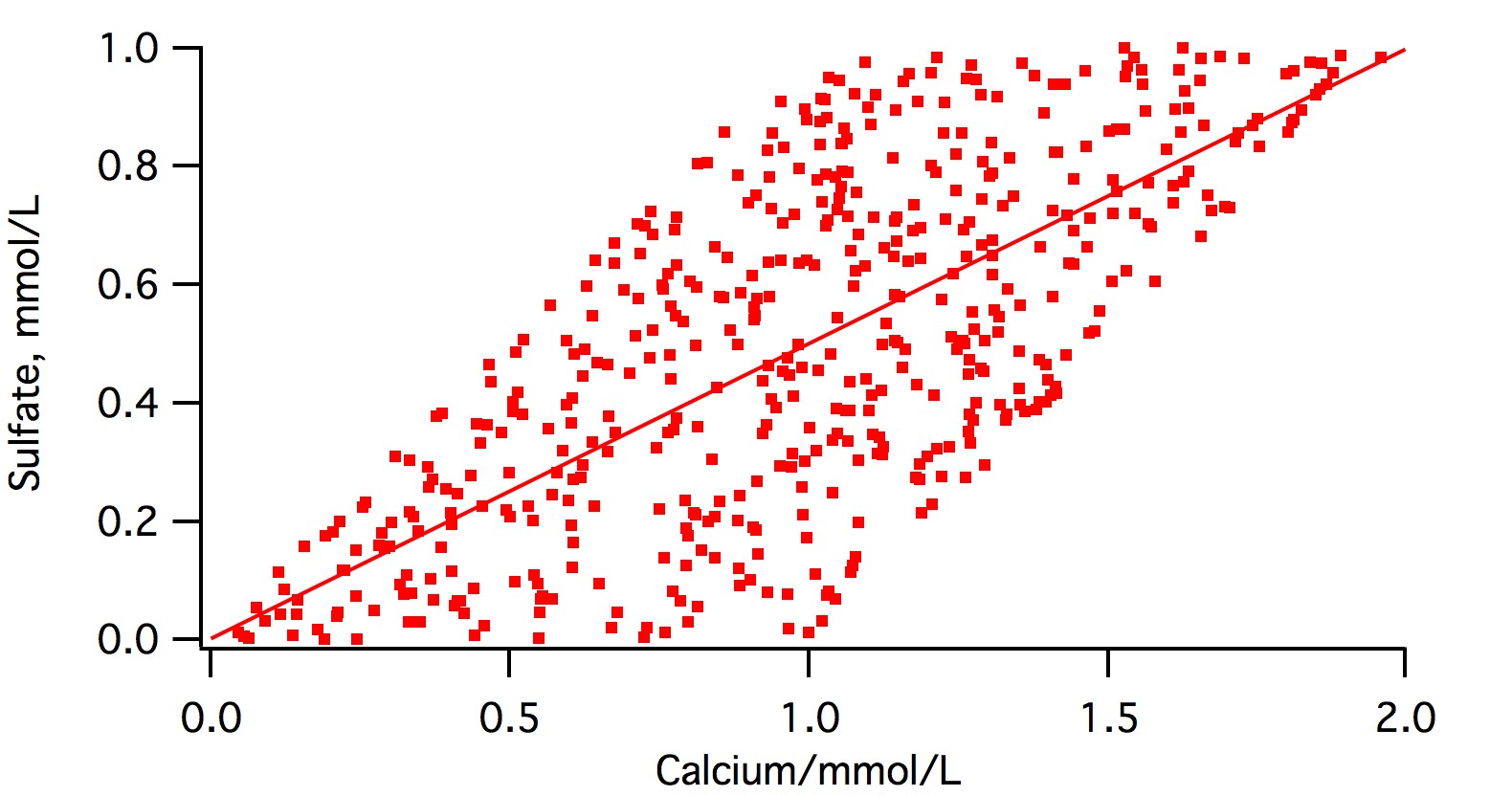
My own experience with natural waters has been that the majority of alkalinity will be associated with calcium and magnesium,
That would certainly be expected where most of the mineral content in the water comes from dissolution of limestone or dolomitic limestone by carbonic acid and, of course, that is extremely common.
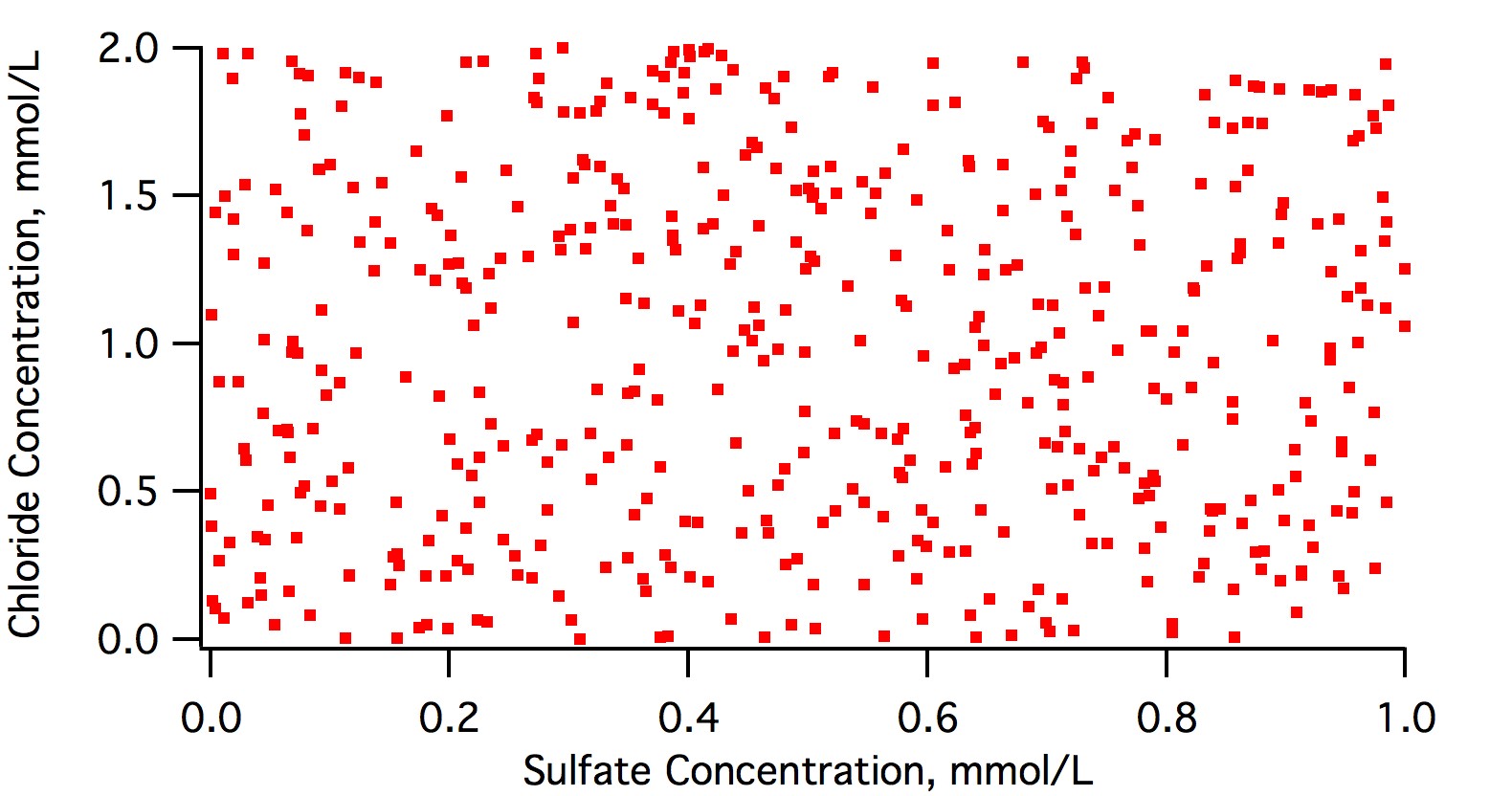
the majority of chloride with sodium
This is often the case near the sea and where roads are salted in winter. But you will find chloride not associated with any cation where the utility has adjusted pH with HCl.
as well as most sodium with chloride,
Consider the Searless Valley (California) whose biggest town is named Trona (Na2CO3). In regions like that where there were once alkaline lakes (note that OP is near San Diego) I think you will probably find sodium "paired" with carbonate and sulfate. I put "paired" in quotes because the point I am trying to make is that you can't pair ions. All you can say is that the total number of positive charges must equal the number of negative. Did a sodium ion come from dissolved mineral mirabilite (paired with sulfate) or from dissolved trona (paired with bicarbonate/carbonate) or dissolved halite (paired with chloride) or from sodium hydroxide (paired with OH-) added by the utility to adjust pH upwards for mains protection or from sodium hypochlorite (paired with OCl-) added to chlorinate the water or from an ion exchange process (replacing calcium or magnesium)?
while sulphate is usually found with calcium or magnesium.
As calcium, magnesium and sodium are the only three cations found in any appreciable concentration in potable water there is a good chance that sulfate will be found with one of these present. Where there is sodium present, as there would be where groundwater from a xeric region is used in a xeric region (mirabilite present) it shouldn't be surprising to find it paired with sodium (if it is present - it's not in OP's water).
Each won't exactly balance with one another,
In gypseous water i.e. water where there is permanent an temporary hardness (Burton, Dortmund, Viernna) they won't even come close to balancing as the calcium and magnesium is 'shared' between sulfate and bicarbonate.
As a general observation, you cannot tell what salts (minerals) were responsible for a particular ion composition. It's easy enough to determine how much of each of the 7 ions of interest are produced by given quantities of the dozen or so minerals that might be involved but going he other direction is not easy to do as it would, using my numbers, involve solving 12 equations in 7 unknowns.
but if they don't by some large measure, as in this case, it can indicate there could have been human intervention. Could some part of your water supply be softened, and if it might, could it be somewhere in your control?
If one sees a water report with appreciable alkalinity and/or sulfate but no hardness one can conclude that there was intervention (exchange softening) but in this case there is certainly no support for that conclusion. I certainly don't know the details of San Diego's water supply but given where it is located I am certainly not surprised to find a fair amount of sodium in it.
I'm thinking that more likely natural water would be calcium at 75ppm, Mg 4, Na 6, SO4 3, Cl 9 and alkalinity 199, which run through a low efficiency ion exchange water softener could produce what you have. It may well be better with more calcium rather than sodium.
OTOH as I don't know the details of what San Diego does (they were experimenting with desalinization and even treated gray water at one point) I can't say that this is not a possibility. According to their web sites they source 85 - 95% of their water from sources outside the region. They give a list of the operations they perform (the usual sedimentation, flocculation, filtration and pH adjustment) but do not mention ion exchange.
I'll file that one away for future reference. AMS is not in their catalogue but is, perhaps, available on special order.








































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)