butterpants
Well-Known Member
- Joined
- Apr 29, 2013
- Messages
- 1,146
- Reaction score
- 128
I'm always looking for a challenge and tend to get bored easily once my mind feels as if it has conqured something. Just a fact of life for me.... so sours and wild yeast brewing is next on my list to tackle. Problem is it takes so damm long to ferment out... so I need a brewing related project to keep my mind stimulated in the meantime.
I want to put together a microbiological quality control lab for my brewing. Currently in the process obtaing hardware and textbooks to teach it to myself. I've looked over what Jamil and Chris say in Yeast and seen some of the documentation the Brewing Science Institute (which is like 30 minutes from my house) puts out for equipment. Was wondering if any of you guys are doing this and had suggestions on....
1) Microscope models
2) Autoclave alternative
3) Microbiology Textbooks
4) Staining/testing kits n media
5) Source for lab odds n ends
6) Hemocytometer
Before someone jumps in and says "go back to school" or something similar.... I was in a molecular biology PhD program back in the 90ies and left to go take a job with more interesting prosspects. Most of the lab technology and techniques are familiar eventhough molecular and microbiology aren't much alike. I'd like to be able to test and identify all my produced beers for wild yeast and bacterial contamination and also be able to isolate and produce pure cultures on a small scale.
I want to put together a microbiological quality control lab for my brewing. Currently in the process obtaing hardware and textbooks to teach it to myself. I've looked over what Jamil and Chris say in Yeast and seen some of the documentation the Brewing Science Institute (which is like 30 minutes from my house) puts out for equipment. Was wondering if any of you guys are doing this and had suggestions on....
1) Microscope models
2) Autoclave alternative
3) Microbiology Textbooks
4) Staining/testing kits n media
5) Source for lab odds n ends
6) Hemocytometer
Before someone jumps in and says "go back to school" or something similar.... I was in a molecular biology PhD program back in the 90ies and left to go take a job with more interesting prosspects. Most of the lab technology and techniques are familiar eventhough molecular and microbiology aren't much alike. I'd like to be able to test and identify all my produced beers for wild yeast and bacterial contamination and also be able to isolate and produce pure cultures on a small scale.



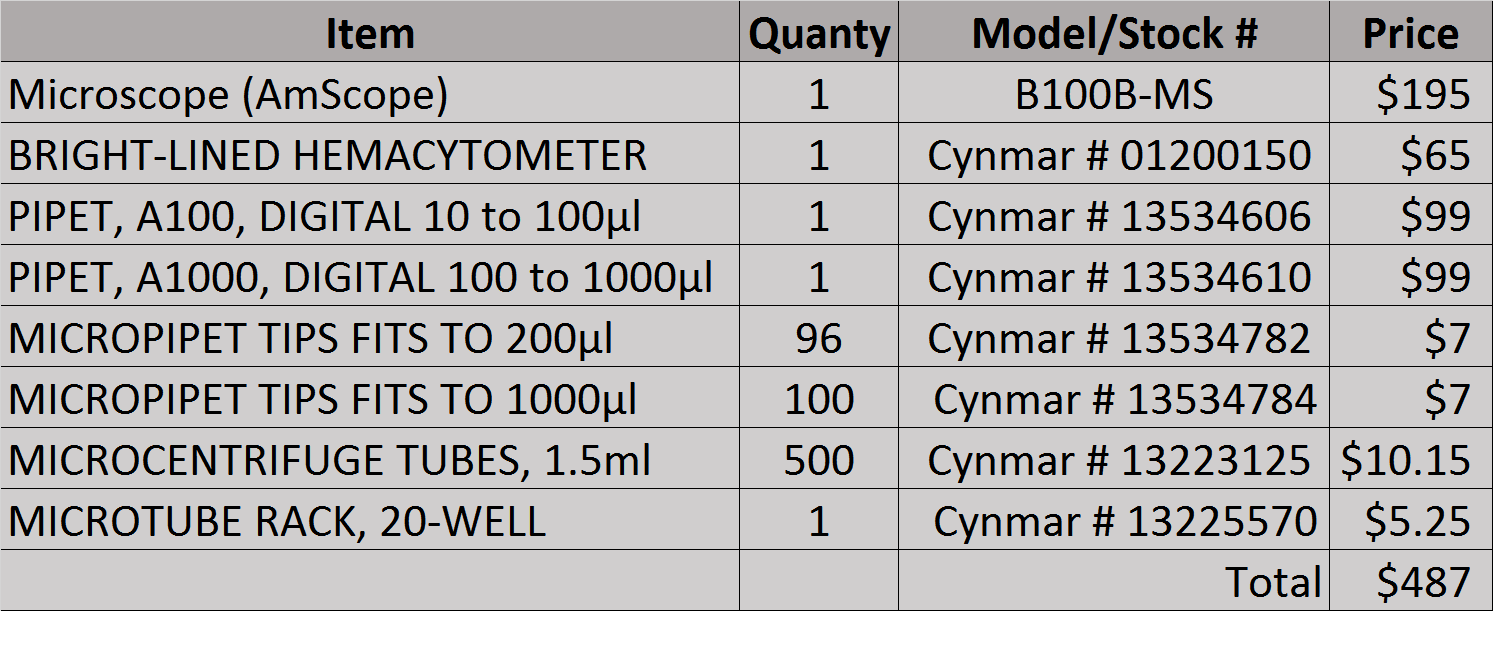


![IMG_0194[1].jpg IMG_0194[1].jpg](https://cdn.homebrewtalk.com/data/attach/191/191294-IMG-0194-1-.jpg)
![IMG_0196[1].jpg IMG_0196[1].jpg](https://cdn.homebrewtalk.com/data/attach/191/191295-IMG-0196-1-.jpg)
![IMG_0208[1].jpg IMG_0208[1].jpg](https://cdn.homebrewtalk.com/data/attach/191/191296-IMG-0208-1-.jpg)
![IMG_0212[1].jpg IMG_0212[1].jpg](https://cdn.homebrewtalk.com/data/attach/191/191297-IMG-0212-1-.jpg)

