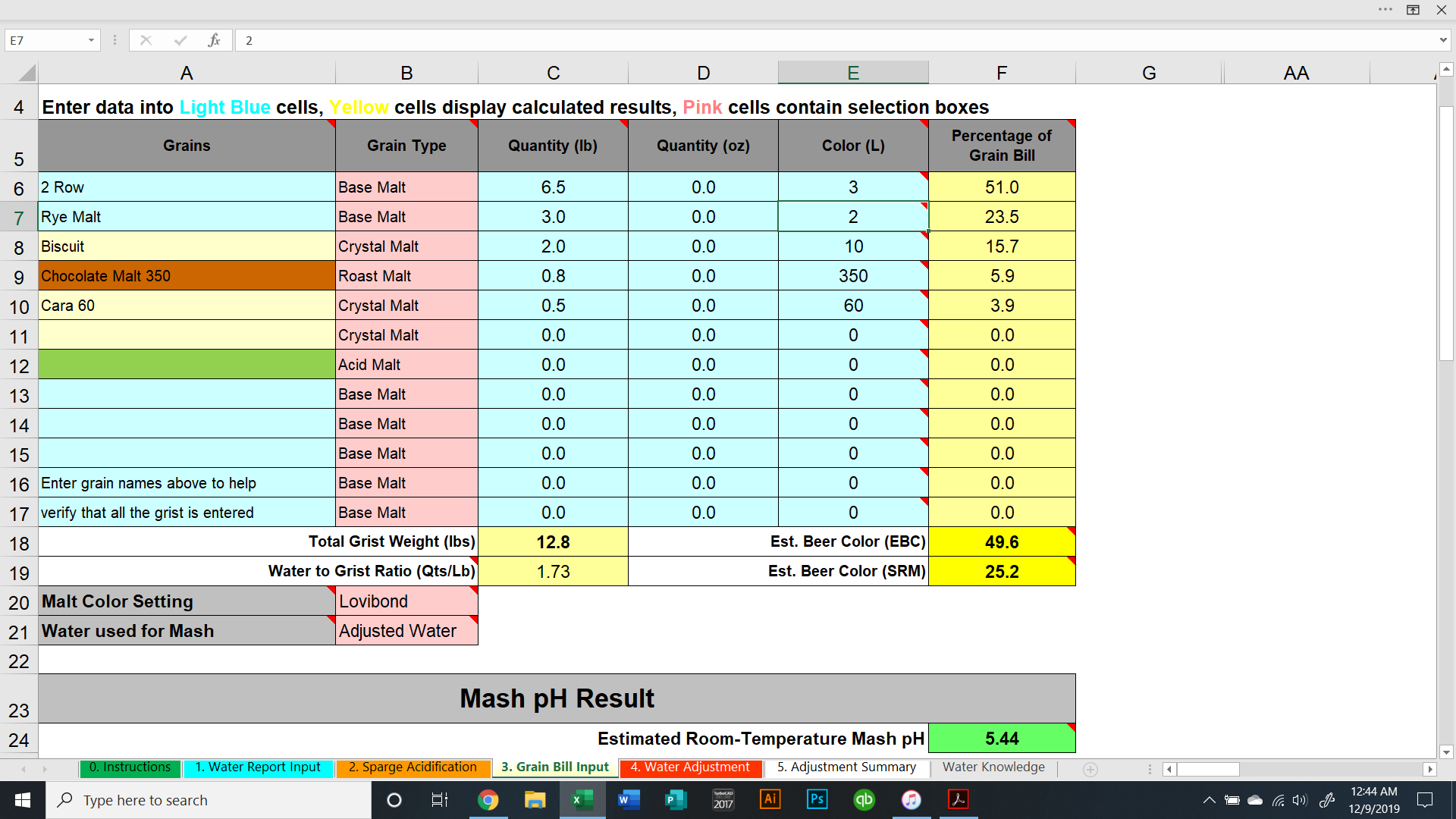
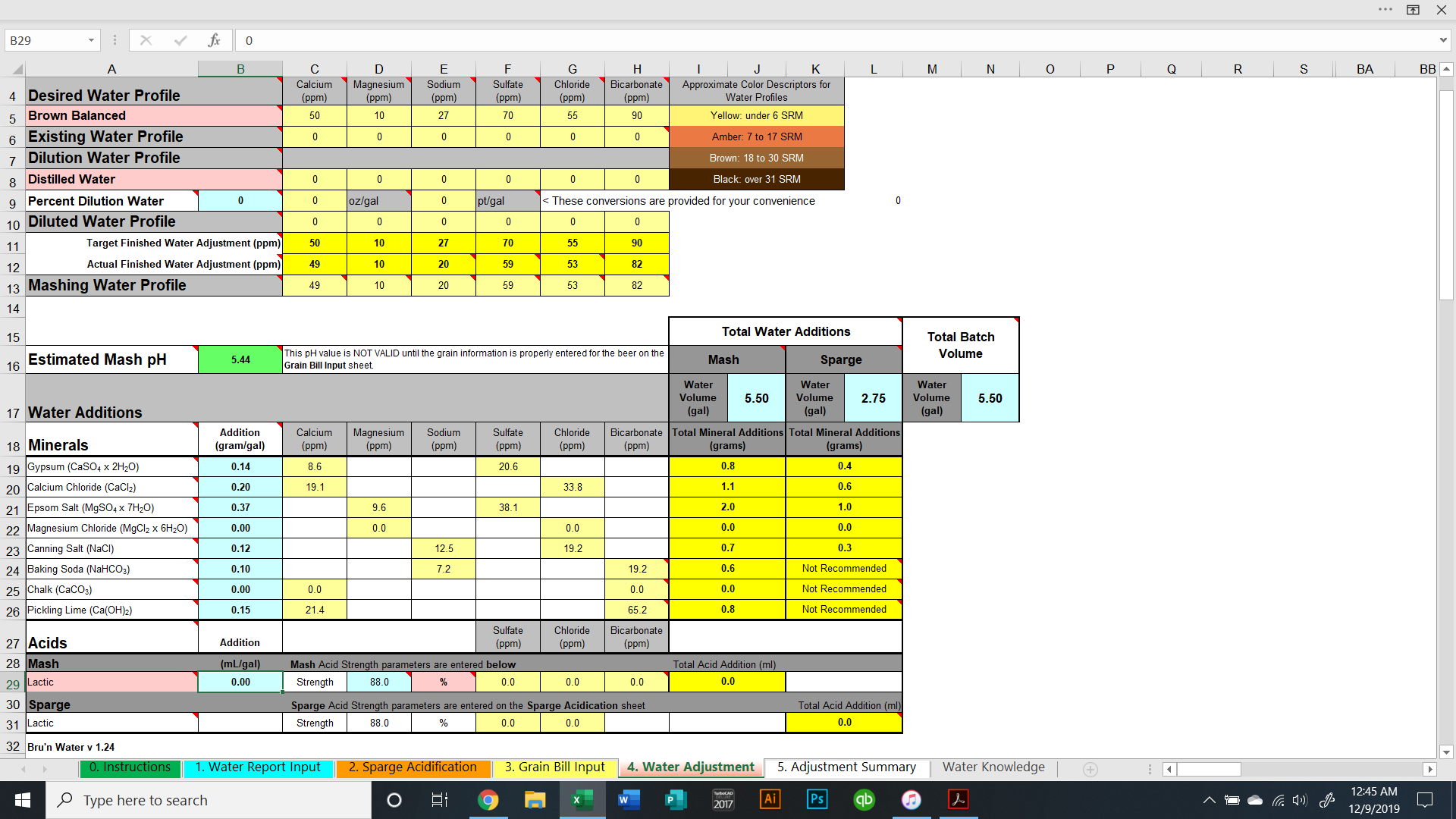
I'm brewing a brown ale next weekend, 11/30. I'm working on the water additions and I'm comparing Bru'n Water to Mash Made Easy.
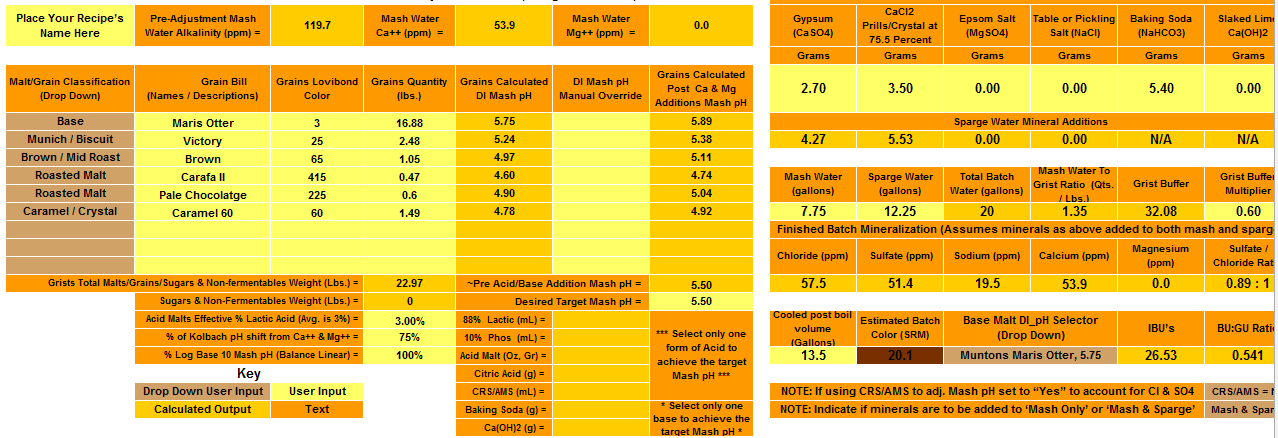
The values are significantly different comparing the two programs. To achieve a pH of 5.5, Bru'n water says to add 9.3g baking soda and Mash Made Easy is saying to add 5.8 grams baking soda. If I change Bru'n Water to say 5.8g baking soda, it drops the pH to 5.33.
They're significant differences and I am just not sure which one to use. Screenshots are shown below.
Am I missing something in either sheet? (Screenshots below and sheets attached).
Previously, I was thinking Bru'n Water was not accurate when it came to darker beers (no offense @mabrungard). Is Bru'n Water accurate when it comes to dark beers? Does anyone have any real life measurements showing that it's accurate?
Same question applies to Mash Made Easy. Does anyone have any measured proof that MME is accurate with dark beers?
Thanks for the help.
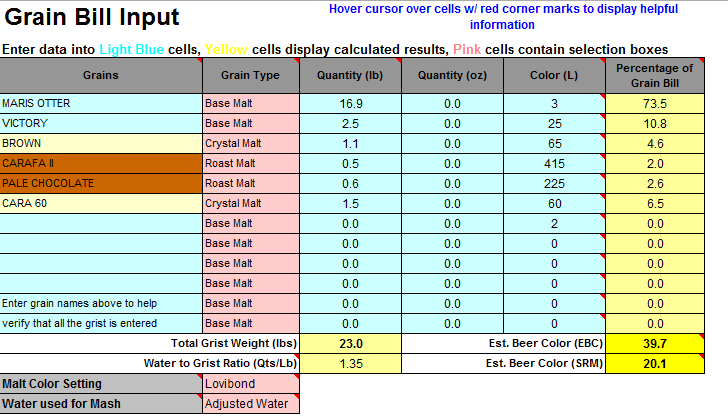
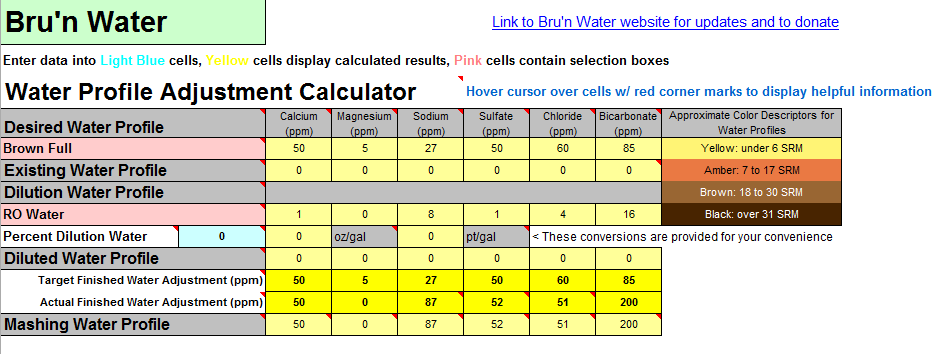
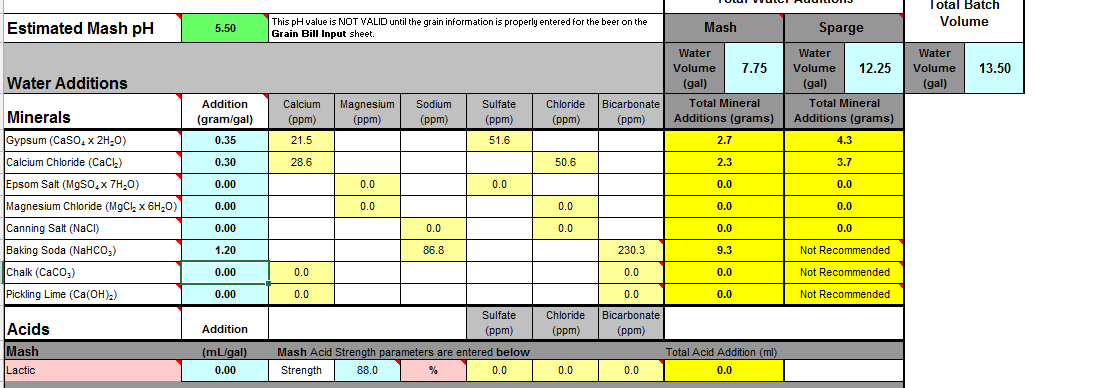
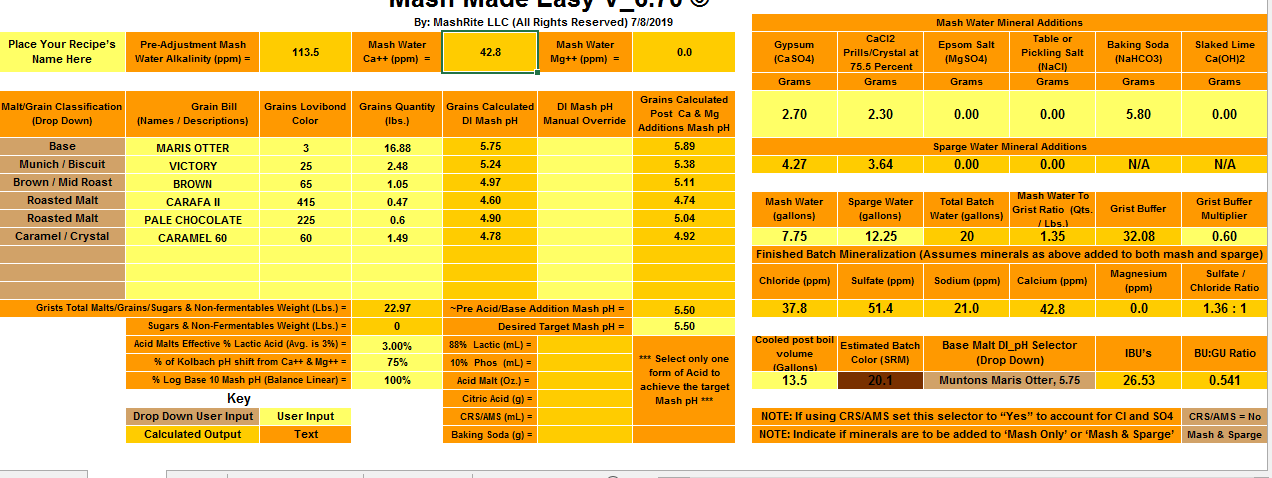
The values are significantly different comparing the two programs. To achieve a pH of 5.5, Bru'n water says to add 9.3g baking soda and Mash Made Easy is saying to add 5.8 grams baking soda. If I change Bru'n Water to say 5.8g baking soda, it drops the pH to 5.33.
They're significant differences and I am just not sure which one to use. Screenshots are shown below.
Am I missing something in either sheet? (Screenshots below and sheets attached).
Previously, I was thinking Bru'n Water was not accurate when it came to darker beers (no offense @mabrungard). Is Bru'n Water accurate when it comes to dark beers? Does anyone have any real life measurements showing that it's accurate?
Same question applies to Mash Made Easy. Does anyone have any measured proof that MME is accurate with dark beers?
Thanks for the help.





























![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)