Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Changes include:
1) Retains all of the improvements made in version 8.20
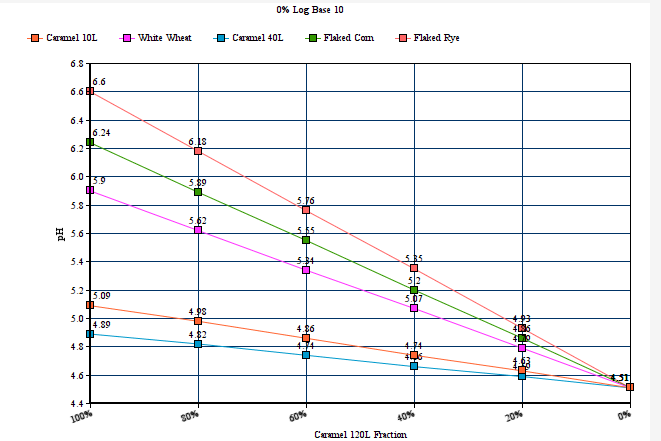
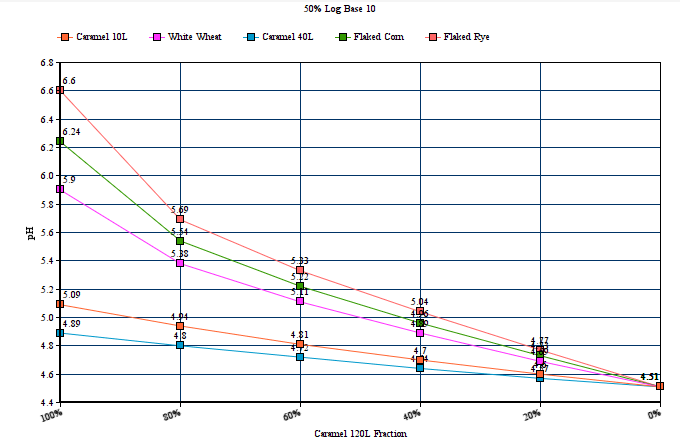
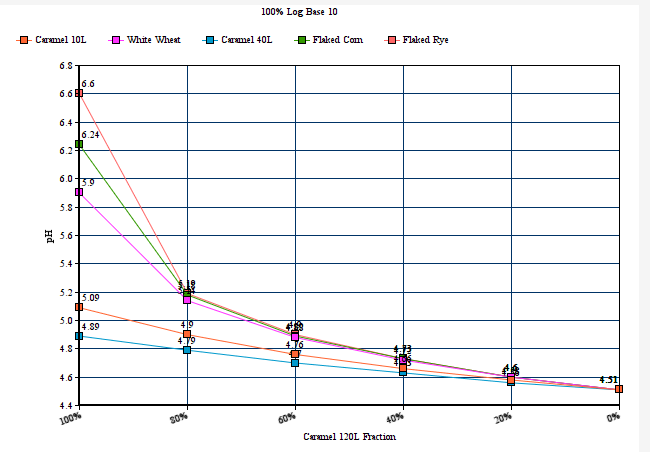
2) Restores end user selectability of the percentage by which 'MME' computes via logarithmic and linear slope functions. In keeping with dark/robust grists which are rich in deep roasted and caramel/crystal malts, and for which many home brewers have observed the need to add lots of baking soda or calcium hydroxide to hit their mash pH target, the 100% logarithmic setting generally seems to work best here, but to more closely mirror the output of a number of other well known mash pH assistant calculators, setting this selectors percentage to 40% log based ( give or take a bit) brings MME's output at the deep roasted end (and all across the color or grist scale) well in line with the others. The option is there to dial in MME to best fit with your personal findings and experience, as well as to more closely mirror some other popular calculators output advice if this is your desire. There are merits to both approaches, and the jury is still out, but if/when a verdict is reached, MME will comply with a number of the collective others at a setting of around 40%.
3) Fixes a nagging issue whereby for all earlier releases the computed need for an addition of baking soda or calcium hydroxide automatically inserted baking soda's sodium into the ppm (mg/L) output display. This version does not update the sodium mineralization and/or calcium mineralization boost from these two pH raising minerals until after the user adds the requested pH raising quantity to the mash water "Mineral Additions" section in the upper right of the main page. This allows one to better see what is happening to mineralization, as well as to allow for split additions of baking soda and calcium hydroxide, or to not add these minerals at all, or to add more or less of them than MME has computed (at your choice). The former way in which MME handled this was a hold-over from before calcium hydroxide was an option, leaving only baking soda as a choice, and thus the automatic sodium boost seen from it.
1) Retains all of the improvements made in version 8.20
2) Restores end user selectability of the percentage by which 'MME' computes via logarithmic and linear slope functions. In keeping with dark/robust grists which are rich in deep roasted and caramel/crystal malts, and for which many home brewers have observed the need to add lots of baking soda or calcium hydroxide to hit their mash pH target, the 100% logarithmic setting generally seems to work best here, but to more closely mirror the output of a number of other well known mash pH assistant calculators, setting this selectors percentage to 40% log based ( give or take a bit) brings MME's output at the deep roasted end (and all across the color or grist scale) well in line with the others. The option is there to dial in MME to best fit with your personal findings and experience, as well as to more closely mirror some other popular calculators output advice if this is your desire. There are merits to both approaches, and the jury is still out, but if/when a verdict is reached, MME will comply with a number of the collective others at a setting of around 40%.
3) Fixes a nagging issue whereby for all earlier releases the computed need for an addition of baking soda or calcium hydroxide automatically inserted baking soda's sodium into the ppm (mg/L) output display. This version does not update the sodium mineralization and/or calcium mineralization boost from these two pH raising minerals until after the user adds the requested pH raising quantity to the mash water "Mineral Additions" section in the upper right of the main page. This allows one to better see what is happening to mineralization, as well as to allow for split additions of baking soda and calcium hydroxide, or to not add these minerals at all, or to add more or less of them than MME has computed (at your choice). The former way in which MME handled this was a hold-over from before calcium hydroxide was an option, leaving only baking soda as a choice, and thus the automatic sodium boost seen from it.
Last edited: