Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
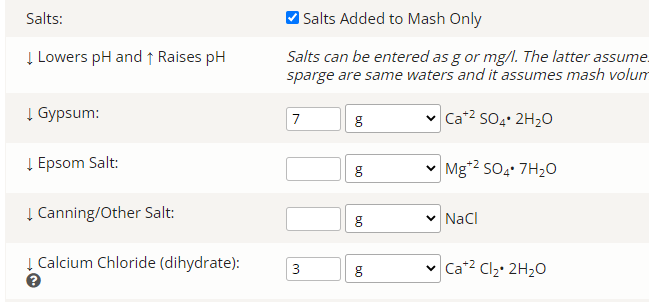
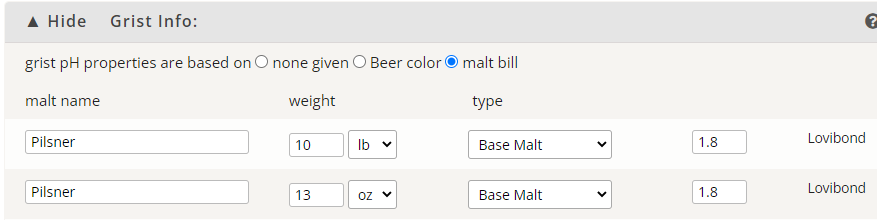
Question for you - not arguing just asking - does 4ml at 88% sound normal to you?
I could only attempt to 'nominally' (as opposed to specifically) answer your question if I had every pertinent detail as to 'source' water analyticals, mineral additions to same, water volumes (with emphasis upon the mash, but also with respect to the sparge), grain and adjunct details, and grain and adjunct weights.








![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)

















































