Big Monk
Trappist Please! 🍷
- Joined
- Dec 24, 2015
- Messages
- 2,192
- Reaction score
- 1,154
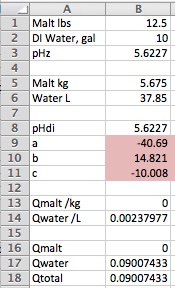
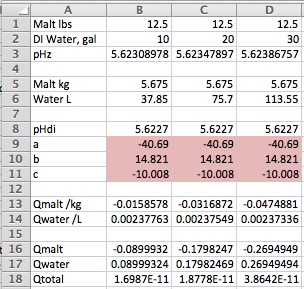
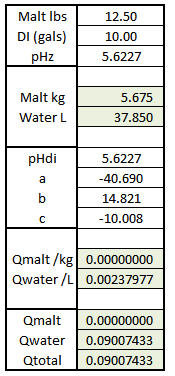
Reading along I was thinking this very same thing when this post came along. The basics here are really very simple to the point that the first spreadsheet I did that predicted mash pH accurately I put together during the presentation before mine at a MBAA meeting because I wansn't very interested in what the guy was saying but noted that he did include a spreadsheet in his talk and was thus able to illustrate his points and I figured my talk would benefit from that too. To use a simple spreadsheet, though, you have to have complete malt data and you aren't going to get that from the maltsters which means you have to get it from someone like me and a few other guys who are willing to make the measurements for you or do it yourself and as there is no ASBC sanction for this there is no MOA and so the data would not be standardized. In addition to having complete malt data you have to accept the 'model' represented by that data. If you don't have that data then you have to deduce it from what you do have which is information on malt type and malt color. Augmenting that data by giving the brewer the ability to enter pHDi is obviously a step in the right direction. Giving him the ability to put in the first (linear) buffering coefficient too is another step in the right direction and allowing him to enter the second order coefficient another. But now we expect him to understand what these terms mean or rather at least what the effect of modifying them might be and that's way beyond most potential users of a spreadsheet or program - even among professional brewers.
So I think it's fantastic that you guys are exploring all these avenues but you'll probably wind up where I did: 255 rows and 30 columns for the water sheet and 134 rows by 16 columns for the malt sheet. There's quite a lot of empty cells in there, though. It's got all kind of neat bells and whistle's of course and I can do almost anything water related with it but I stopped offering it to people years ago and would never consider doing so again (though I have given it to a few people).
I’ve set a goal for myself for this week to put a 31 Row/26 column sheet together of my current build along with volume and gravity for brewday. I’m of the opinion that if you are entering the grain bill anyway for pH estimation, you may as well track gravity and volume as well.





















































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)