Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
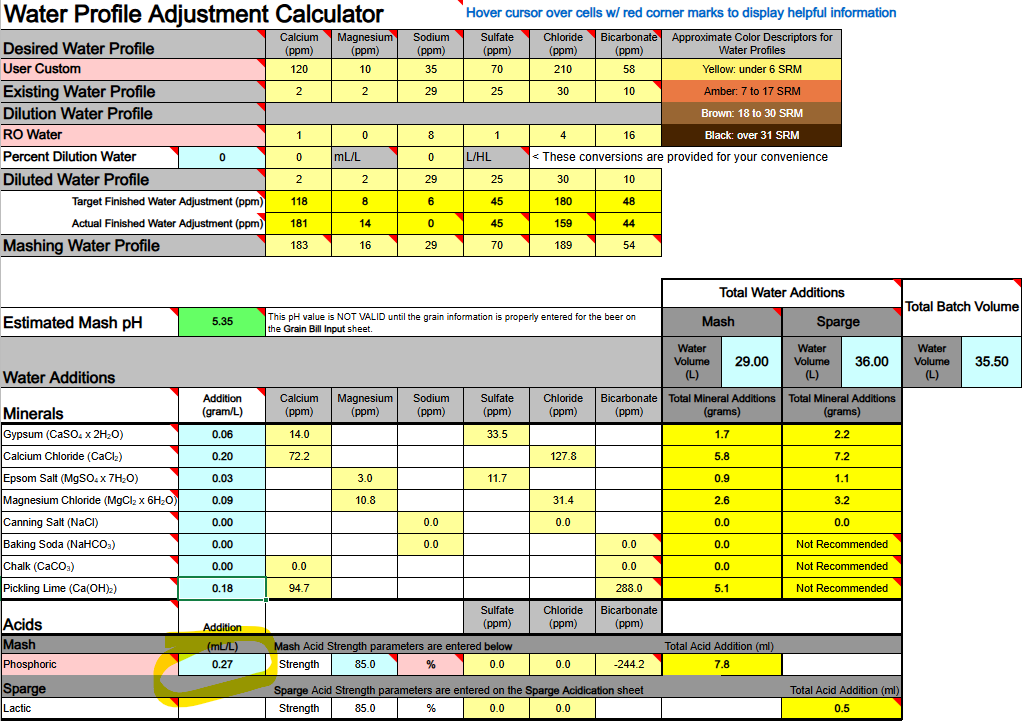
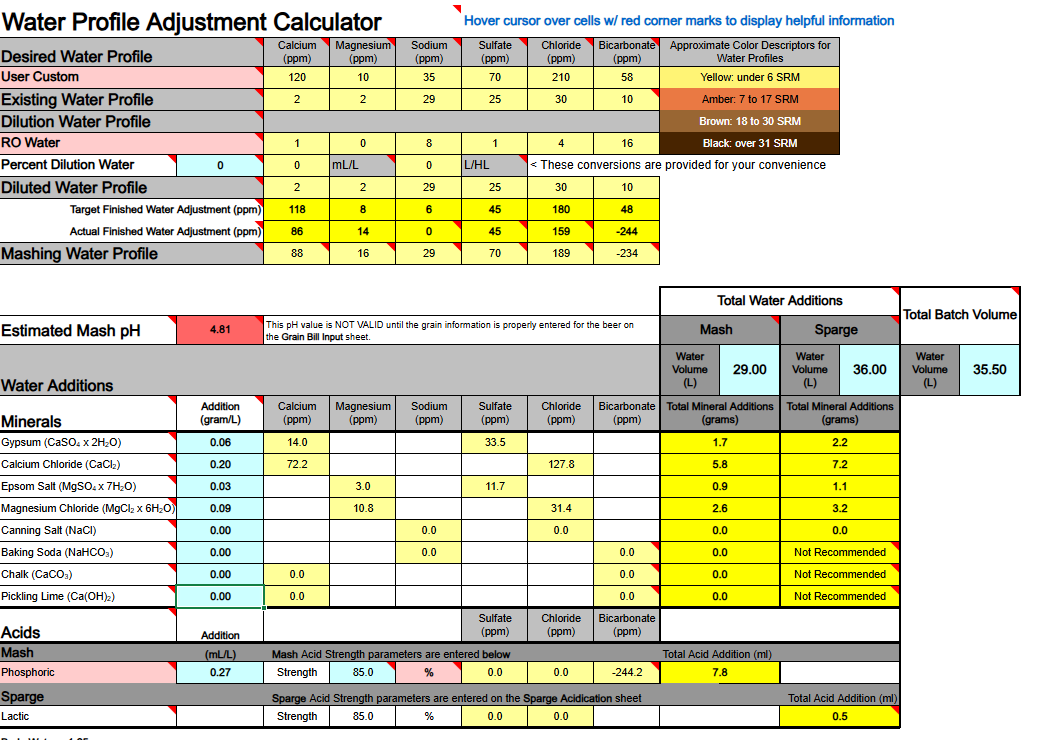
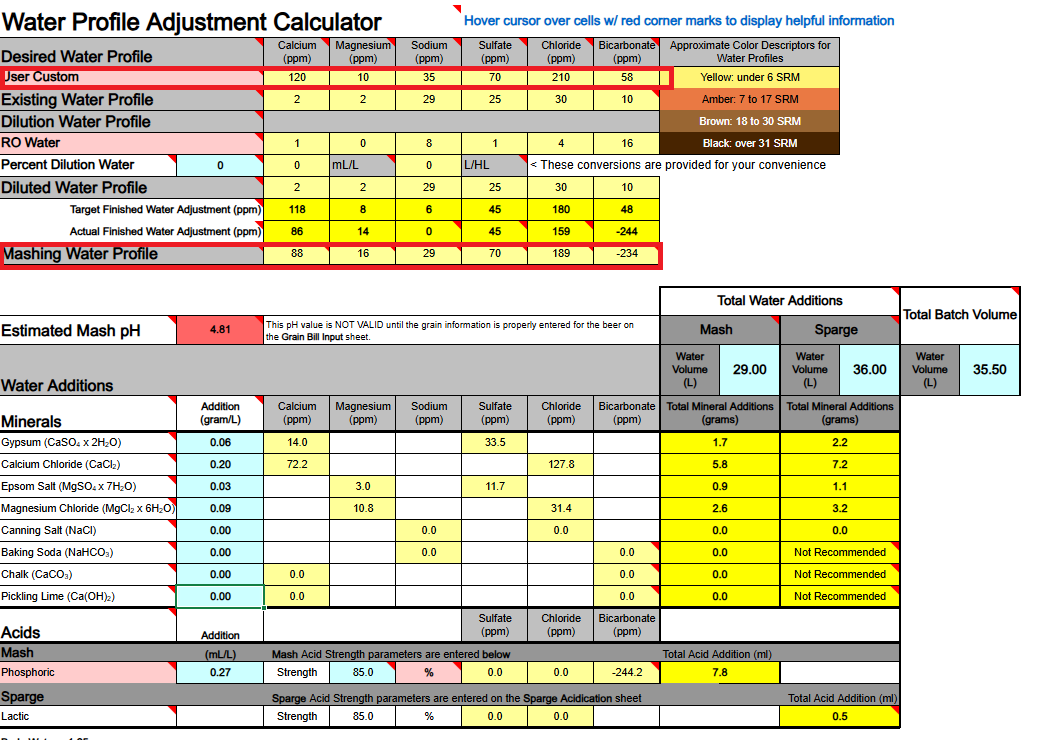
1) Calcium Hydroxide should only be used to both add Ca++ calcium ions and raise water pH if you are using DI, RO, or Distilled water as your source. If your source water contains Bicarbonate ions (HCO3-) the bicarbonate ions will bind to the Ca++ ions and remove them as a highly insoluble precipitate of Calcium Carbonate (CaCO3) which mostly drops out. Until most of your bicarbonate is removed via precipitation as CaCO3 you will not accomplish the adding of Ca++ ions to your water.
Ca(OH)2 + HCO3- → CaCO3↓ + H2O + OH-
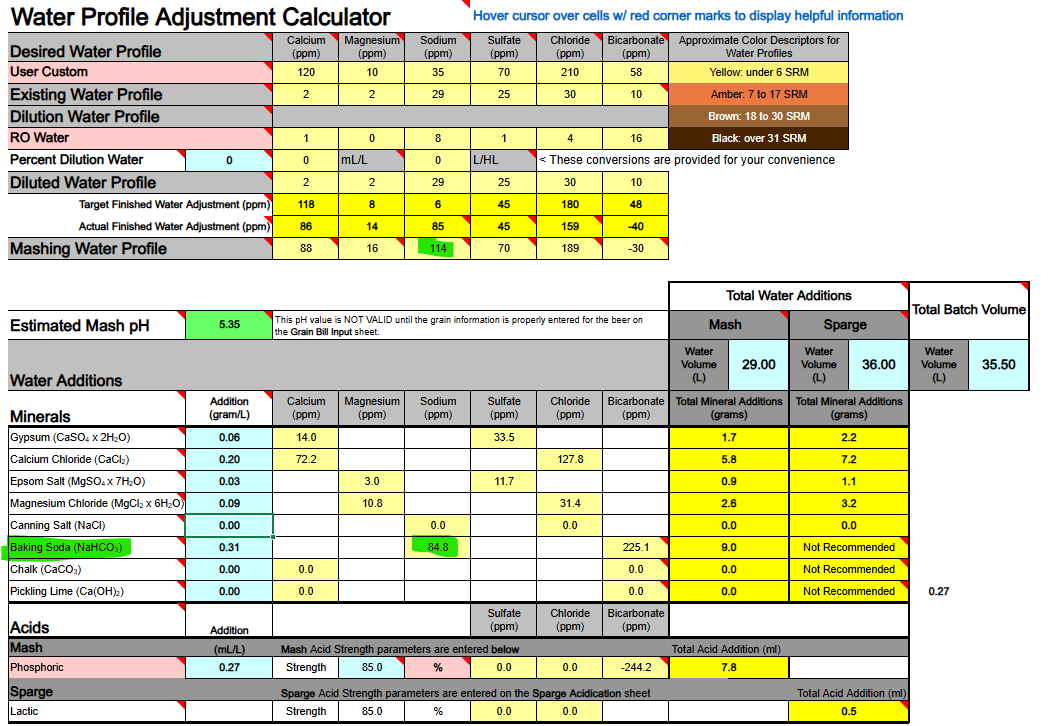
2) If you are thinking about adding both Baking Soda (NaHCO3) and Calcium Hydroxide whereby to both add calcium and sodium, and also raise your waters pH, the very same issue will hinder and bite you, so never mix these two minerals as part of your mineralization routine, even if you are starting with DI, RO, or Distilled water as your source.
Ca(OH)2 + NaHCO3 → CaCO3↓ + H2O + NaOH
3) Any dissolved CO2 within your water, even if it is sourced from DI, RO, or Distilled, will combine with the calcium in Ca(OH)2 to form CaCO3 and drop out the calcium until nearly all CO2 is removed.
Ca(OH)2 + CO2→ CaCO3↓ + H2O
All of the above has me questioning the overall viability of using Ca(OH)2 whereby to add Ca++ calcium ions to your brewing water or to raise its pH. It does not appear to me (subject to correction) that any current mash pH assistant software is factoring in any of the above reactions. It's probably best to simply avoid using Ca(OH)2. Certainly don't use it if your water source is not DI, RO, or Distilled. It is not easily or readily predictable as to the actual outcome of its use.
Ca(OH)2 + HCO3- → CaCO3↓ + H2O + OH-
2) If you are thinking about adding both Baking Soda (NaHCO3) and Calcium Hydroxide whereby to both add calcium and sodium, and also raise your waters pH, the very same issue will hinder and bite you, so never mix these two minerals as part of your mineralization routine, even if you are starting with DI, RO, or Distilled water as your source.
Ca(OH)2 + NaHCO3 → CaCO3↓ + H2O + NaOH
3) Any dissolved CO2 within your water, even if it is sourced from DI, RO, or Distilled, will combine with the calcium in Ca(OH)2 to form CaCO3 and drop out the calcium until nearly all CO2 is removed.
Ca(OH)2 + CO2→ CaCO3↓ + H2O
All of the above has me questioning the overall viability of using Ca(OH)2 whereby to add Ca++ calcium ions to your brewing water or to raise its pH. It does not appear to me (subject to correction) that any current mash pH assistant software is factoring in any of the above reactions. It's probably best to simply avoid using Ca(OH)2. Certainly don't use it if your water source is not DI, RO, or Distilled. It is not easily or readily predictable as to the actual outcome of its use.
Last edited:






















![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)