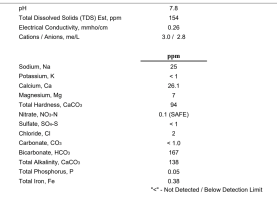
I just got my water report back from Ward's lab and I'm a bit surprised it has changed as much as it has. I'm on a well and I knew it would, but it was still a bit surprising. We even have an iron filter. I am a bit disappointed as I really didn't want to have to buy water for beer. Adjusting salts, sure, but I brew too often to want to buy water. None the less, I think I am going to start using distilled water and just eliminate any possibly the water is causing my issues. I'm relatively new so I want to tackle independent things slowly, one issue at a time, so I am looking at the best water additions. A happy middle ground for all beer styles that I can just get used to adding and expand upon for specific beer styles after getting familiar with it. I also do not like super hoppy beers, I like my beer to taste like malt, but don't want to be drinking syrup either (I really dislike Imperial stouts for instance).
Do I understand distilled water correctly in that it is a clean slate and has been stripped of its mineral content? Distilled water essentially starts at zero's across the board and 7 pH?
What are your suggestions for salt additions? Is there a mix that I can purchase that just has a bit of everything so I just add the one?
Is adding the middle of the recommended amounts for all salt additions (in Palmer's book) a good starting point?
I have attached my profile if anyone is curious.
Side note: If you've read my other post(s), this may answer some of the issues re: sweetness and the weird after taste I can't shake?
Do I understand distilled water correctly in that it is a clean slate and has been stripped of its mineral content? Distilled water essentially starts at zero's across the board and 7 pH?
What are your suggestions for salt additions? Is there a mix that I can purchase that just has a bit of everything so I just add the one?
Is adding the middle of the recommended amounts for all salt additions (in Palmer's book) a good starting point?
I have attached my profile if anyone is curious.
Side note: If you've read my other post(s), this may answer some of the issues re: sweetness and the weird after taste I can't shake?