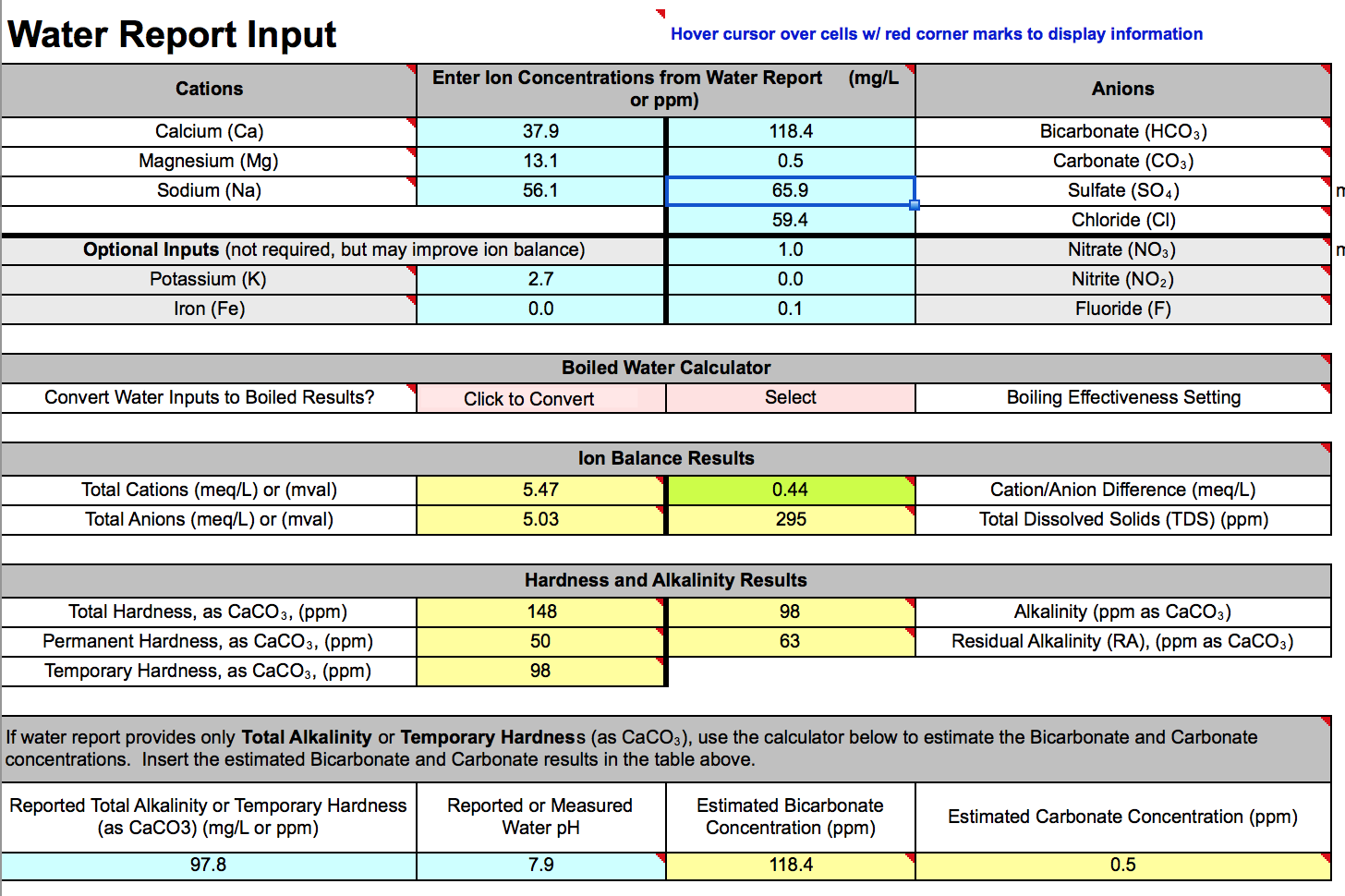
Hi, this is my first post, and thanks for reading. I am new to water chemistry, as will be apparent I assume. I am using the supporter version on Bru'n Water to both learn and prep for modifying my water.
My water is from a city source that can have dramatic swings depending on time of year and sources being leveraged. I have spoken at length with one of the city water engineers to make sure I am reading the water report correctly, and am using the best values possible, however, when I put the information into the program, I am getting an ion imbalance of .44 (meq/L), while the spreadsheet says at .5 difference we should be questioning the the report. I have double checked the units to make sure I am not using ppb.
SO on to the first questions, is there any point in trying to get a closer balance of ions? Can this much of an imbalance throw the pH prediction off by .5 to .6 points?
To see what is happening in the real world, I made a "mini mash" equal to 10% of the planned mash. At 10 minute intervals I took pH readings and after the 20 minute mark, my mash stabilized at a pH of 5.3. I am using an Aspera PH20 (which I now realize has an accuracy of +/- .1 so I may have to upgrade at some point). The program predicts a mash pH of 5.89
I am hoping to get some suggestions on were to look to understand the discrepancy, since I am clearly doing something wrong here. If anyone wants to see the water report or the sheets, I am happy to post them.
Thanks for any input / advice!
My water is from a city source that can have dramatic swings depending on time of year and sources being leveraged. I have spoken at length with one of the city water engineers to make sure I am reading the water report correctly, and am using the best values possible, however, when I put the information into the program, I am getting an ion imbalance of .44 (meq/L), while the spreadsheet says at .5 difference we should be questioning the the report. I have double checked the units to make sure I am not using ppb.
SO on to the first questions, is there any point in trying to get a closer balance of ions? Can this much of an imbalance throw the pH prediction off by .5 to .6 points?
To see what is happening in the real world, I made a "mini mash" equal to 10% of the planned mash. At 10 minute intervals I took pH readings and after the 20 minute mark, my mash stabilized at a pH of 5.3. I am using an Aspera PH20 (which I now realize has an accuracy of +/- .1 so I may have to upgrade at some point). The program predicts a mash pH of 5.89
I am hoping to get some suggestions on were to look to understand the discrepancy, since I am clearly doing something wrong here. If anyone wants to see the water report or the sheets, I am happy to post them.
Thanks for any input / advice!