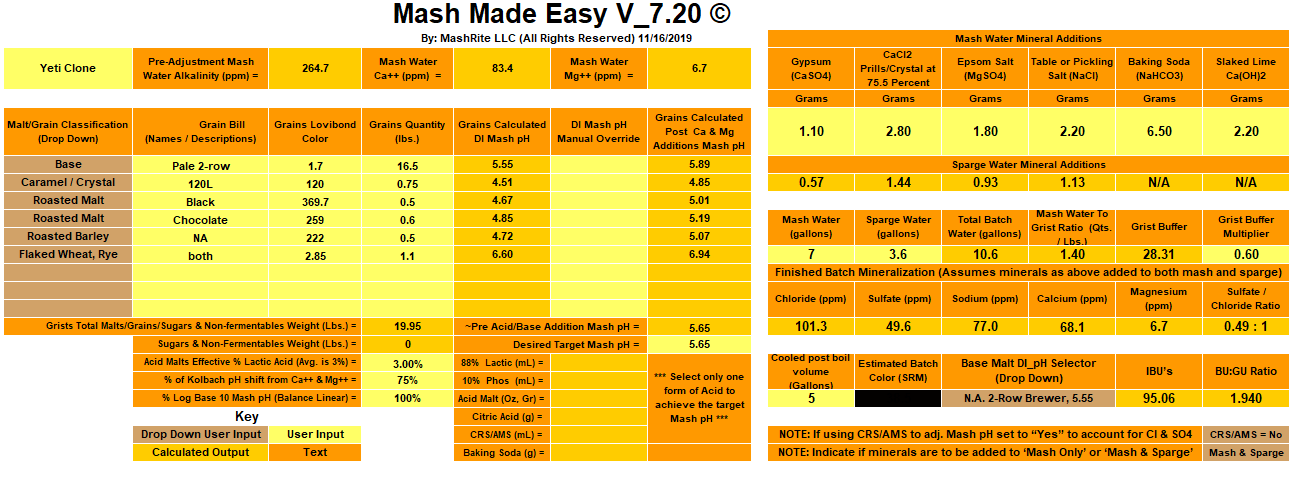
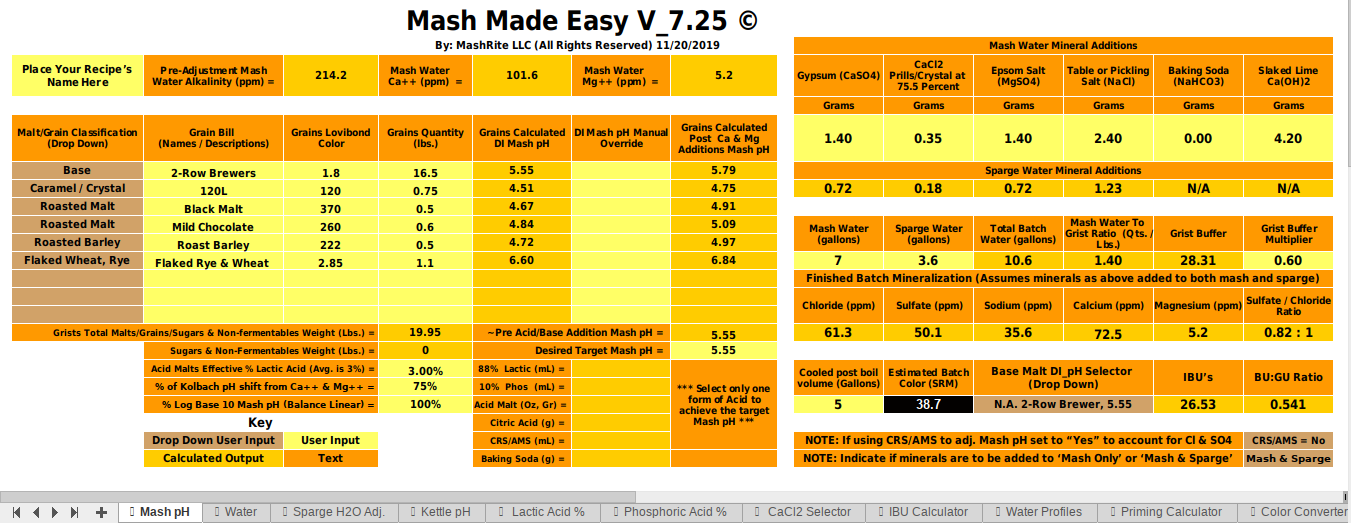
Hi all. I'm looking to do water chemistry adjustments for the first time. I've done a bunch of research and want to make sure I have this right.
I'm brewing a 5 gal batch of imperial stout. My target water profile (close to London water) requires a deal more calcium and sodium than my local water offers. I've played with a bunch of different water chem calculators, and all reached the same conclusion: the only way to get my Ca and Na up enough without throwing my sulfates and chlorides all out of whack is to add a bunch of baking soda, and then smaller amount each of Gypsum, Calcium Chloride, canning salt and chalk. This of course raises my mash pH way too high, so according to the calculators, I'll have to add 3 mL of lactic acid (88%) to the mash water and another 1.6 to the sparge (that's 4.6 mL for 10.6 gal total).
Three questions:
I'm brewing a 5 gal batch of imperial stout. My target water profile (close to London water) requires a deal more calcium and sodium than my local water offers. I've played with a bunch of different water chem calculators, and all reached the same conclusion: the only way to get my Ca and Na up enough without throwing my sulfates and chlorides all out of whack is to add a bunch of baking soda, and then smaller amount each of Gypsum, Calcium Chloride, canning salt and chalk. This of course raises my mash pH way too high, so according to the calculators, I'll have to add 3 mL of lactic acid (88%) to the mash water and another 1.6 to the sparge (that's 4.6 mL for 10.6 gal total).
Three questions:
- Does the above sound right/reasonable?
- Is the amount of lactic acid so much that it will affect the taste of the beer?
- How do I add the chalk (only need about 1g, but have heard it does not dissolve)