Hi all - I have lurked here for the better part of a year. Decided to join and this is my first post here. I've recently gotten back into brewing after about a 10 yr hiatus. Since reentering the hobby I've brewed about 20 all grain batches. To give you an idea of my process, I brew on a Grainfather, use a ferm chamber to control temps, and I keg.
Just this weekend I took my first taste of an XPA that I brewed (it's actually the Resilience recipe to help support those impacted by the Australian wildfires, scaled down to my system and adapted for ingredients I have access to). The beer was well carbonated and tasted nice and refreshing. However, there was a rather pleasant, but unexpected tartness to the beer...almost sour. I actually really like it.
What concerns me is - this beer may have over attenuated. Here's my recipe/notes from the brew:
Two additional relevant factst: 1) I went to a different homebrew store for grains this day. Grain crush appeared to be finer than what I get at my LHBS. This could have thrown off my numbers due to increased efficiency. And 2) I recently took a can of my beer into my LHBS just because I talk with one of the guys there about recipes and wanted to share a recent batch he helped select the hops for. He said he'd sample at the end of the day, and I did not return for a couple weeks. When I brought it up, he didn't remember much, but said it was good. The only knock he had, was that it was tart. Now, I don't recall this beer being tart at all. I had canned this beer to empty a keg, so I do have some left in the fridge to test out myself.
I know that's a lot of info - but I wanted to provide enough detail for a proper evaluation. Really what I am wondering is what I need to do moving forward. Could this be that my lactic acid additions are reaching flavor thresholds? Is this just a LAB contamination, or do I have a bigger problem? I am meticulous about cleaning and sanitation, so I am a bit confused how this happened (although I did have my dad helping me that day and he is not a brewer). I ferment in PET carboys - do I need to clean using a special technique? Or replace?
I am also wondering if all of my beers have been tart, and maybe I am just not that sensitive to it. I have ordered a pH meter and plan to test the beer in question as well as one of the remaining cans that I shared with the guy at the LHBS. Any input to help me figure this out is much appreciated! Thanks for reading.
Just this weekend I took my first taste of an XPA that I brewed (it's actually the Resilience recipe to help support those impacted by the Australian wildfires, scaled down to my system and adapted for ingredients I have access to). The beer was well carbonated and tasted nice and refreshing. However, there was a rather pleasant, but unexpected tartness to the beer...almost sour. I actually really like it.
What concerns me is - this beer may have over attenuated. Here's my recipe/notes from the brew:
Water:
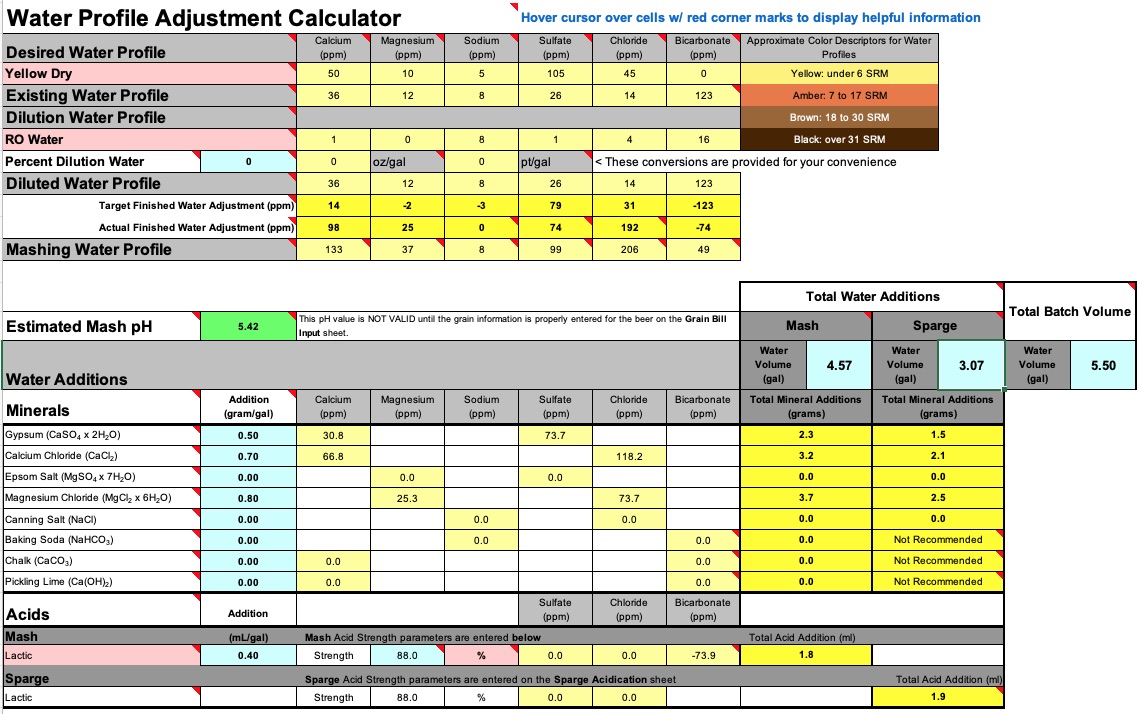
Adjusted my water using Bru'n Water tool. Used 7.64 gal between strike and sparge. Used a campden tab to treat water prior to heating. Targeted final values were:
Grain Bill:
Mash at 150F for 60. Mash out at 167F for 10 min. Boil for 60 min.
Vital Stats:
*I use a Tilt and just take manual hydrometer readings to confirm OG/FG readings. Tilt readings confirmed my OG reading, but measured a 1.007 FG. My hydrometer (admittedly a cheap one), on a degassed (12 hrs) sample measured 1.005.Adjusted my water using Bru'n Water tool. Used 7.64 gal between strike and sparge. Used a campden tab to treat water prior to heating. Targeted final values were:
- Sulfate: 99 ppm
- Chloride: 206 ppm
- Magnesium: 37 ppm
Grain Bill:
- 9.75 lb 2-row
- 1 lb Vienna
- 0.5 lb White Wheat Malt
Mash at 150F for 60. Mash out at 167F for 10 min. Boil for 60 min.
- 60 min - 0.3 oz Simcoe
- 15 min - 0.3 oz Cashmere, 2 oz Mosaic
- 10 min - 4.4g yeast nutrient, whirlfloc tab
- 5 min - 0.7 oz Cashmere
Vital Stats:
- System efficiency is in the low to mid 70s.
- Target batch size: 5.5 gal
- Actual: 5.25 gal in fermenter
- Target OG: 1.049
- Actual OG: 1.052
- Target FG: 1.009 (assuming 82% attenuation, upper end of Omega's stated range)
- Actual FG: 1.005 or 1.007*
Two additional relevant factst: 1) I went to a different homebrew store for grains this day. Grain crush appeared to be finer than what I get at my LHBS. This could have thrown off my numbers due to increased efficiency. And 2) I recently took a can of my beer into my LHBS just because I talk with one of the guys there about recipes and wanted to share a recent batch he helped select the hops for. He said he'd sample at the end of the day, and I did not return for a couple weeks. When I brought it up, he didn't remember much, but said it was good. The only knock he had, was that it was tart. Now, I don't recall this beer being tart at all. I had canned this beer to empty a keg, so I do have some left in the fridge to test out myself.
I know that's a lot of info - but I wanted to provide enough detail for a proper evaluation. Really what I am wondering is what I need to do moving forward. Could this be that my lactic acid additions are reaching flavor thresholds? Is this just a LAB contamination, or do I have a bigger problem? I am meticulous about cleaning and sanitation, so I am a bit confused how this happened (although I did have my dad helping me that day and he is not a brewer). I ferment in PET carboys - do I need to clean using a special technique? Or replace?
I am also wondering if all of my beers have been tart, and maybe I am just not that sensitive to it. I have ordered a pH meter and plan to test the beer in question as well as one of the remaining cans that I shared with the guy at the LHBS. Any input to help me figure this out is much appreciated! Thanks for reading.