I'm not going to go all in with everything you can do with water, I've read through the articles and it's just to much for me - right now.
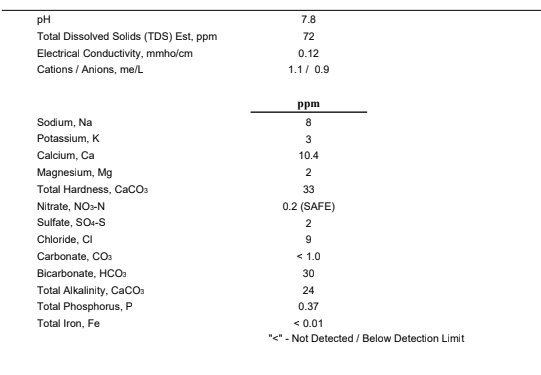
I did however believe I'd ask my town about what they measure and see how that stacks up...of course it's not a Ward report...but what are your thoughts on what they did give me?
I did however believe I'd ask my town about what they measure and see how that stacks up...of course it's not a Ward report...but what are your thoughts on what they did give me?
- Calcium (Hardness CACO3) 20-45 mg/L; our water is considered “soft.”
- Sodium 6.0-6.0 mg/L
- Chlorine- we are a Free Chlorine Disinfection System; we do not use Chloramines.
- Chlorine Residual 1.30-1.60 mg/L
- Chlorite 0.071-0.454 mg/L
- Chlorine Dioxide nd- 0.048 mg/L
- Chlorine Residual 1.30-1.60 mg/L
- Total Alkalinity 20-30 mg/L