So as you guessed I'm having trouble with my water and there is a lot of info out there. I am trying to wrap my head around it.
Can you let me know if I going about this the right way?
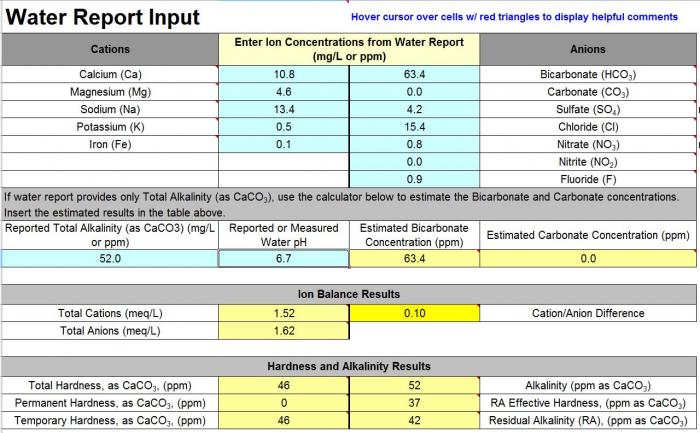
Here is my water profile:
Calcium 10.8
Magnesium 4.6
Sodium 13.4
Potassium 0.5
Iron 0.1
Sulfate 4.2
Chloride 15.4
Nitrate 0.8
Nitrite 0
Alkalinity is 52
PH is 6.7
Brew'n water calculates Bicarbonates at 63.4 and Carbonate at 0
Chlorine levels are at 1.5
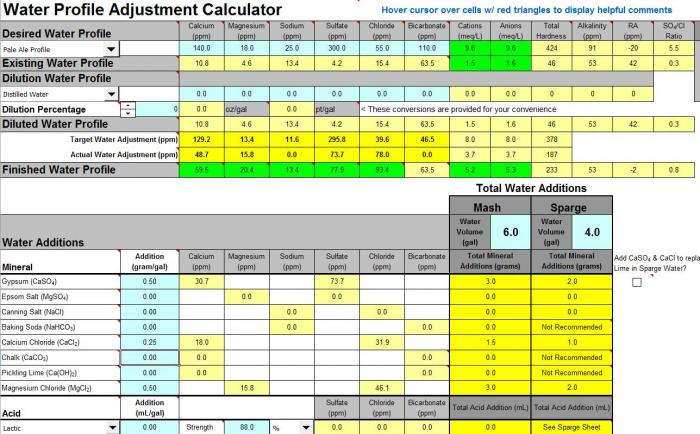
So this seems soft and low on PH based on other things I've read but when I plug a grain bill into Brew'n Water my mash PH is still too high
I made some water adjustments to fit the pale ale profile and mash ph is 5.6. Would it be better to get that lower?
Also, I am going to use campden tablets for my next brew to take out the chlorine, but what flavor does the chlorine add to your beer. Could this be the culprit?
I am going to send a sample to get tested and see if the results match up.
Can you let me know if I going about this the right way?
Here is my water profile:
Calcium 10.8
Magnesium 4.6
Sodium 13.4
Potassium 0.5
Iron 0.1
Sulfate 4.2
Chloride 15.4
Nitrate 0.8
Nitrite 0
Alkalinity is 52
PH is 6.7
Brew'n water calculates Bicarbonates at 63.4 and Carbonate at 0
Chlorine levels are at 1.5
So this seems soft and low on PH based on other things I've read but when I plug a grain bill into Brew'n Water my mash PH is still too high
I made some water adjustments to fit the pale ale profile and mash ph is 5.6. Would it be better to get that lower?
Also, I am going to use campden tablets for my next brew to take out the chlorine, but what flavor does the chlorine add to your beer. Could this be the culprit?
I am going to send a sample to get tested and see if the results match up.




