bobo31
Well-Known Member
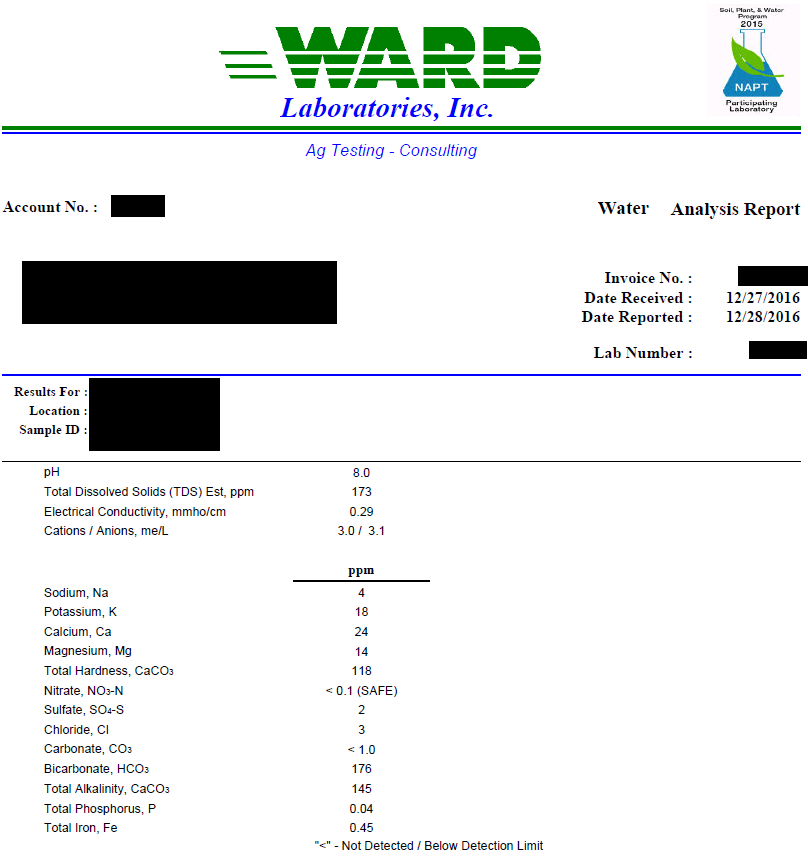
For those that live in southern Maryland and use well water do you have to alter your water? I assume we are all on the same aquifer and have pretty much the same water traits. I have not had my water tested yet, so I am wondering what your conditions might be. My water seems to be hard. Is there a place around here that does free water testing? I have an RO filter so diluting tap water is an option or if need be using only RO water is an option. Since I am new to this hobby I don't have the experience to tell if my water conditions could improve my results.
Off topic, but I saw the Hop Heads are meeting this Saturday. I can't make this meeting but I hope to be at the next one.
Thank you for any advice.
Off topic, but I saw the Hop Heads are meeting this Saturday. I can't make this meeting but I hope to be at the next one.
Thank you for any advice.