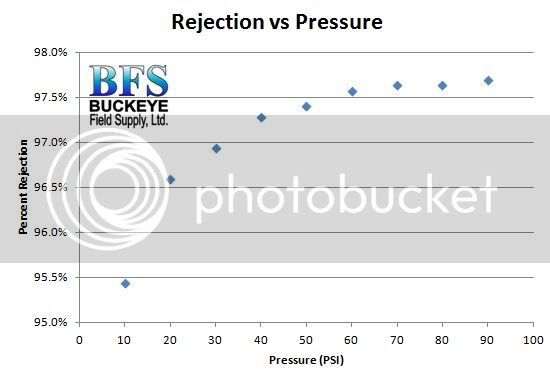
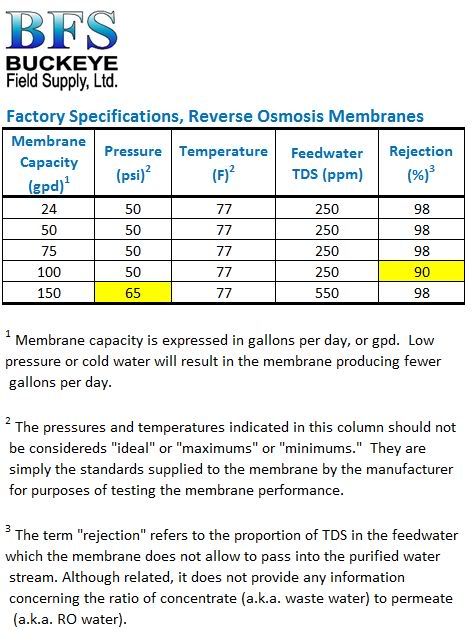
Recognize that the rejection rate for differing ions varies for any RO or nanofiltration process. Monovalent ions like sodium and chloride are smaller in diameter and pass through the membrane pores easier. The larger divalent ions like Ca, Mg, and SO4 do not pass as easily. They are rejected at a higher rate than the monovalent ions.
In the case above, the ion-exchange softener is taking out the divalent ions and inserting monovalent. The rejection rate of the RO machine suffers a bit from that. But the good thing about this move is that ions like Na and K are much less likely to precipitate onto the membrane and prematurely clog it. So there is definitely a benefit to pretreating a very hard water via ion-exchange prior to RO feed. You do end up with a little more sodium in the RO product water though. Its a small price to pay. Na at a concentration of less than 40 ppm is generally OK, although 20 ppm is better. If the TDS reading is correct, there is little chance that the Na is more than about 10 ppm.
You are good to go regarding the likely RO water quality.
Membrane processes are very much affected by water temperature. Cold feed water will significantly reduce the efficiency of a RO unit. This is due to the increased viscosity of the cold water. Sure, the rejection rate improves slightly. However, the water production efficiency suffers more. Warming the feed water is very helpful. Keeping your RO unit indoor is an important feature if you live in a cold region.