14thstreet
Well-Known Member
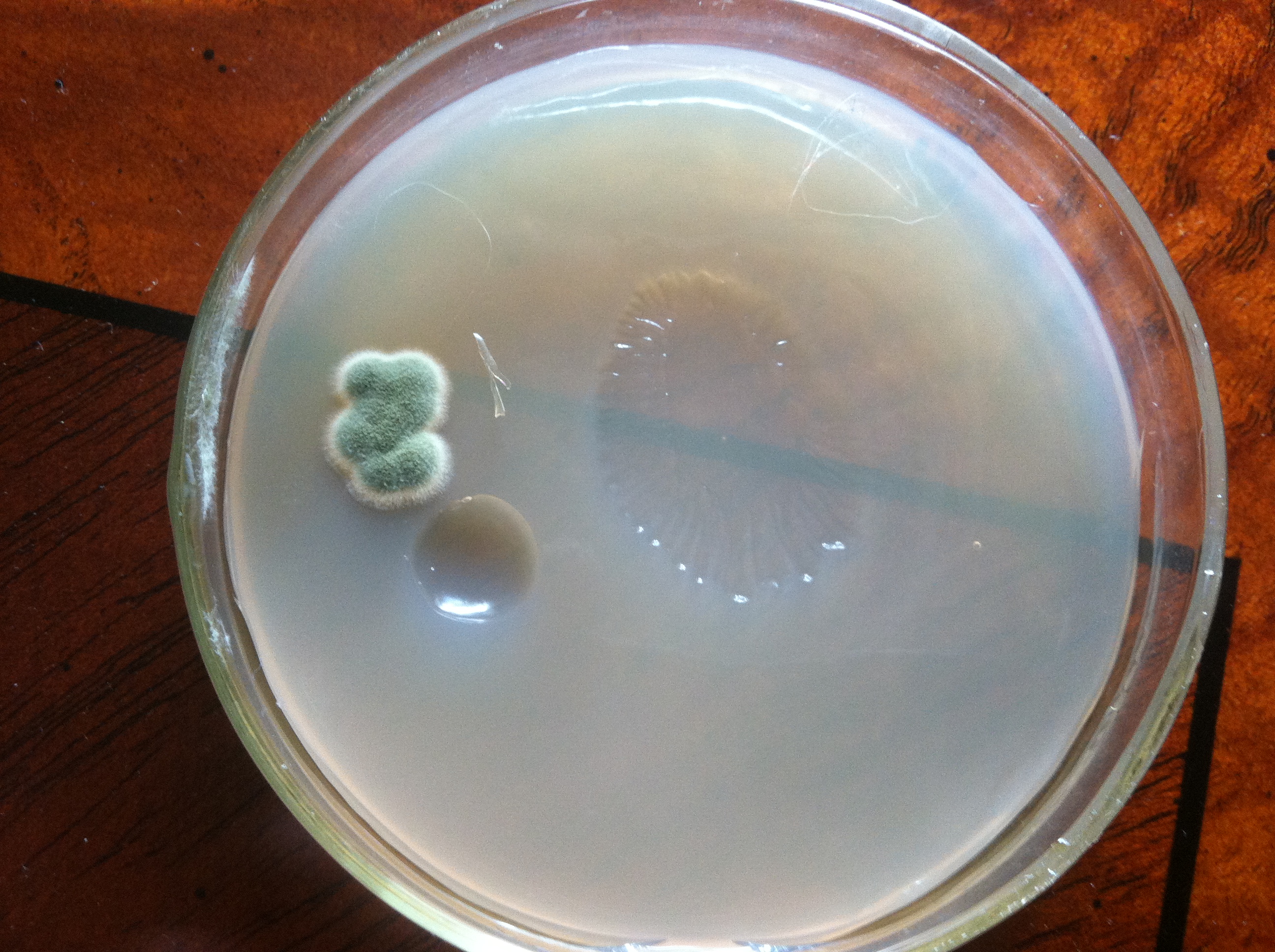
Picture 2 is a harvest from Lakefront Brewery's Wisconsinite from earlier this summer. Looks very different from 3068 and is much cleaner. Viability was determined from a different sample and was 6%. I might try stepping this culture up to see how it goes.
I used this culture in a 1 gallon batch and it went all sour/acetic on me. Nothing from the starter or the fermented batch looked bacterial, so maybe I got some wild yeast in there or the Jeremy King strain went wild.
I'm tuned in, let see some bacteria dance