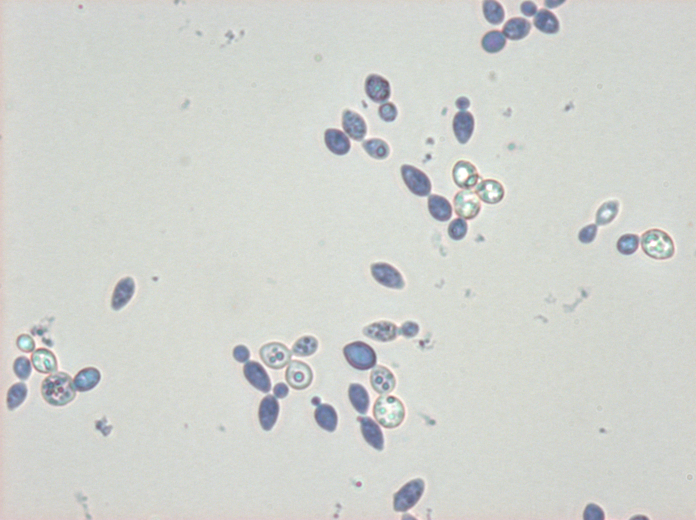
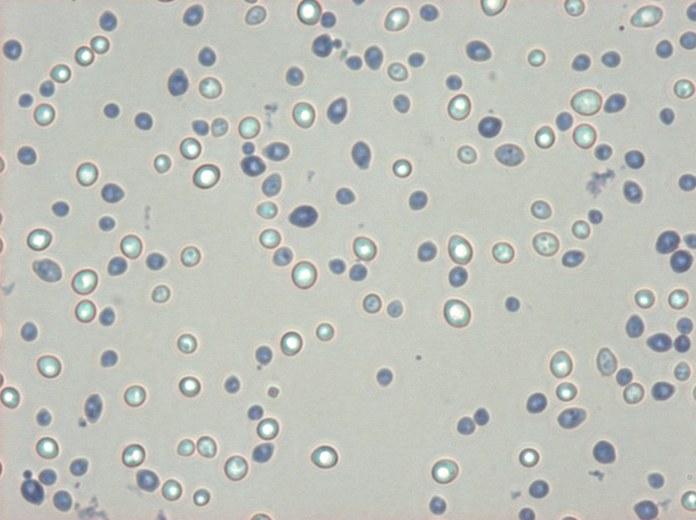
For anyone who is interested: Some Wyeast 3787 under high magnification DIC, no stain.
Very cool. DIC gives amazing contrast. Do the images always look dark like that? That looks like a lot of magnfication... ~ 1000x. What type of scope is that? Was the objective an oil immersion?
(At this moment I'm looking at the bacteria in my own spit, plus some alarmingly large single-cell things that I have no name for; in truth, I can't name anything I see under a scope except for yeast cells