I’ve read in a few threads about passivation of stainless steel. What is this process and why is it necessary? The reason I’m asking is that I have a 20 gallon stainless kettle from 1948 , the ones that hung in the kitchens back when they made real school lunches. Anyways, my pot has a few pits in it and since it’s my boil pot , I’m not worried about infection. Would I need to passivate or not? Thanks 
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Passivation?
- Thread starter Mark3885
- Start date

Help Support Homebrew Talk - Beer, Wine, Mead, & Cider Brewing Discussion Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
Stainless steel is not a homogenous material, there are tiny bits of iron distributed within the nickel and chromium matrix. Exposed iron bits will rust and if not treated can even cause breaches in the kettle. Passivation chemically removes the surface iron and allows the chromium content to grow an oxide layer that can prevent further oxidation of the iron bits.
Bottom line, avoiding kettle breaches is generally A Good Idea and passivation is relatively easy to do: fill the kettle with a 5 to 8% by weight solution of citric acid and water, bring it to 150°F, and hold it there for an hour or more, before draining and rinsing with low mineral water (RO or distilled, for the first rinse)...
and passivation is relatively easy to do: fill the kettle with a 5 to 8% by weight solution of citric acid and water, bring it to 150°F, and hold it there for an hour or more, before draining and rinsing with low mineral water (RO or distilled, for the first rinse)...
Cheers!
Bottom line, avoiding kettle breaches is generally A Good Idea
Cheers!
Thanks that explanation hit the spot . So if my math is correct , I would need about 12.8 lbs of citric acid. Any good sources for citric acid?
Amazon! I just received my latest bag this week (along with brewery stuff we use a teaspoon in the dishwasher to knock down the bicarbonates in our well water and leave the glassware bright).
This is what I get on the regular. There may be larger quantities available.
https://www.amazon.com/dp/B00EYFKNL8
5% acid solution should be adequate if you let it run a while longer...
Cheers!
This is what I get on the regular. There may be larger quantities available.
https://www.amazon.com/dp/B00EYFKNL8
5% acid solution should be adequate if you let it run a while longer...
Cheers!
You could save on citric by using a smaller pot with weights to displace some of the water. Sort of like when people put bricks in their toilet tanks to save water back in the '70s. You only need enough citric solution to make good contact with the interior, and could probably get away with using 5 gallons instead of 20. Use a roaster grate to keep the small pot off the bottom of your BK.
Five Star did a presentation in July that you may be able to access at Passivation and Q and A with Five Star ChemicalsI’ve read in a few threads about passivation of stainless steel. What is this process and why is it necessary? The reason I’m asking is that I have a 20 gallon stainless kettle from 1948 , the ones that hung in the kitchens back when they made real school lunches. Anyways, my pot has a few pits in it and since it’s my boil pot , I’m not worried about infection. Would I need to passivate or not? Thanks
Would running 1 gallon through a CIP for an hour also work?
Brewer Mike
Well-Known Member
- Joined
- Sep 27, 2021
- Messages
- 101
- Reaction score
- 70
How fragile is that oxide layer, and what kind of precautions do you need to take to maintain it?
I would have no idea how to quantify the durability, and I expect there's some dependency on the chemistry and quality of the base stainless steel and the method use for passivation. I have read that citric acid produces a deeper passivation than any other process - even nitric acid - but that's pretty much the extent of what I think I know in that regard.
Between my SS IC and SS big spoon I routinely have metal on metal contact in my boil kettle, which I passivated using citric acid after punching a number of holes in it, yet it has never shown a hint of rust. Same with my MLT and HLT - all three kettles are 20 gallon Blichmann G1 and still look as nice as they perform. I don't really think much about oxide layers in my kettles...
Cheers!
Between my SS IC and SS big spoon I routinely have metal on metal contact in my boil kettle, which I passivated using citric acid after punching a number of holes in it, yet it has never shown a hint of rust. Same with my MLT and HLT - all three kettles are 20 gallon Blichmann G1 and still look as nice as they perform. I don't really think much about oxide layers in my kettles...
Cheers!
- Joined
- Jul 24, 2014
- Messages
- 161
- Reaction score
- 26
For years I passivated with the formerly-recommended high concentration of StarSan, I think it was 1oz per gallon of water right? Apparently, that doesn’t do the trick, so they now say. I need to passivate a fermenter now, which I actually TSP-washed about two months ago. Don’t ask me why, but citric acid makes me very nervous. I’ve dunked my bare hands and arms in normal-concentration StarSan (and SaniClean) countless times over the years, which of course always gives me a little sandpaper/splitting skin in places, but I’ve never worried too much about it. For some reason, citric acid worries me a lot more. I bought some Citrisurf 77 Plus spray, but am still nervous to use it. Is citric acid any more likely to be harmful than StarSan?
I can say an 8% by weight solution at 150°F is hot! but it didn't etch my skin 
I've used it quite a few times to clean up stainless steel stuff like my hop spider and hop blocker, my SS IC, random kitchen equipment, etc, and have never gotten the slightest burn from it or even a tingle. I honestly don't think it's a big deal at all...
Cheers!
I've used it quite a few times to clean up stainless steel stuff like my hop spider and hop blocker, my SS IC, random kitchen equipment, etc, and have never gotten the slightest burn from it or even a tingle. I honestly don't think it's a big deal at all...
Cheers!
- Joined
- Jul 24, 2014
- Messages
- 161
- Reaction score
- 26
That’s good to hear. I probably wouldn’t think a thing of it years ago when I started. Ever since we had kids, I’m suddenly convinced that EVERYTHING is toxic.I can say an 8% by weight solution at 150°F is hot! but it didn't etch my skin
I've used it quite a few times to clean up stainless steel stuff like my hop spider and hop blocker, my SS IC, random kitchen equipment, etc, and have never gotten the slightest burn from it or even a tingle. I honestly don't think it's a big deal at all...
Cheers!
“8% by weight.” Is that the recommendation? What I have is a 7-gallon Chronical. Wondering how to calculate the ratio. I think I’ll get a 6-pound bag of acid from Amazon, for the fermenter, and save the Citrisurf for some other use.
The recommendation I followed was 4 to 10% by weight. I went with 8% rather arbitrarily first time out and stuck with it because it always works 
Water weighs roughly 8.329 pounds per gallon at room temperature...
Cheers!
Water weighs roughly 8.329 pounds per gallon at room temperature...
Cheers!
- Joined
- Jul 24, 2014
- Messages
- 161
- Reaction score
- 26
Sounds like a 6-pound bag from Amazon would take care of 7-gallon fermenter using about 4.5lbs of powder, or 3/4 of the bag. Then the other 1/4 of the bag could mix with 2 gallons of hot water, to dunk fittings and clamps, etc.
8.329lbs x 7gal = 58.3lbs
58.3lbs x .08 = 4.7 lbs of citric acid.
8.329lbs x 2gal = 16.7lbs
16.7lbs x .08 = 1.3 lbs of citric acid
4.7 + 1.3 = 6lbs of citric acid, exactly what comes in that bag.
Sounds like your arbitrary first time formula is perfect for my situation. Nice!
8.329lbs x 7gal = 58.3lbs
58.3lbs x .08 = 4.7 lbs of citric acid.
8.329lbs x 2gal = 16.7lbs
16.7lbs x .08 = 1.3 lbs of citric acid
4.7 + 1.3 = 6lbs of citric acid, exactly what comes in that bag.
Sounds like your arbitrary first time formula is perfect for my situation. Nice!
- Joined
- Jul 24, 2014
- Messages
- 161
- Reaction score
- 26
Just looked at your link above again, and seems like you use a 5lb bag. My mistake,Sounds like a 6-pound bag from Amazon would take care of 7-gallon fermenter using about 4.5lbs of powder, or 3/4 of the bag. Then the other 1/4 of the bag could mix with 2 gallons of hot water, to dunk fittings and clamps, etc.
8.329lbs x 7gal = 58.3lbs
58.3lbs x .08 = 4.7 lbs of citric acid.
8.329lbs x 2gal = 16.7lbs
16.7lbs x .08 = 1.3 lbs of citric acid
4.7 + 1.3 = 6lbs of citric acid, exactly what comes in that bag.
Sounds like your arbitrary first time formula is perfect for my situation. Nice!
Yes, I've been buying 5 pound bags from Amazon, however I did find a 6 pound bag that's considerably cheaper per ounce that I'll switch to on my next buy...
https://www.amazon.com/Granular-Concentrated-Anhydrous-Preservative-Cleaning/dp/B0BXYCL8KP
Cheers!
https://www.amazon.com/Granular-Concentrated-Anhydrous-Preservative-Cleaning/dp/B0BXYCL8KP
Cheers!
- Joined
- Feb 16, 2012
- Messages
- 3,355
- Reaction score
- 4,220
- Location
- Either in the brewery or on the road
Do you treat the acid discharge water with any alkaline to neutralize the pH? I’m on well and septic, but I know that local breweries have to comply with caustic discharges and are required to acidify their caustic cleaning water to neutral before it enters the sewer system.The recommendation I followed was 4 to 10% by weight. I went with 8% rather arbitrarily first time out and stuck with it because it always works
Water weighs roughly 8.329 pounds per gallon at room temperature...
Cheers!
huckdavidson
Well-Known Member
Barkeepers Friend also works for passivation and much more!
https://www.homebrewtalk.com/threads/holy-s-t-bar-keepers-friend.710727/
https://www.homebrewtalk.com/threads/holy-s-t-bar-keepers-friend.710727/
- Joined
- Jul 24, 2014
- Messages
- 161
- Reaction score
- 26
I’ve heard Barkeeper’s Friend is excellent, but that it might remove electronic etching like gallon markings. Otherwise I would love to just use that.Barkeepers Friend also works for passivation and much more!
https://www.homebrewtalk.com/threads/holy-s-t-bar-keepers-friend.710727/
Citric Acid is actually the superior agent for passivation, even superior to Nitric Acid. Oxalic acid (found in BKF) would be somewhere below both.
I am also on a private well and septic system and do not treat the spent Citric Acid solution. I typically do 5 gallons or less, and it goes straight to our 1000 gallon septic tank where I presume dilution and perhaps some chemistry will render it largely inert...
Cheers!
I am also on a private well and septic system and do not treat the spent Citric Acid solution. I typically do 5 gallons or less, and it goes straight to our 1000 gallon septic tank where I presume dilution and perhaps some chemistry will render it largely inert...
Cheers!
Climb
Well-Known Member
Isn’t concentration of the acid important if you are diluting by weight? I didn’t see that concentration was mention on that Amazon link. Is Citric Acid, that is designed for human consumption, limited to some specific concentration?
huckdavidson
Well-Known Member
The deal with BarKeepers friend is that it physically cleans the surface of dirt and physically (and chemically) removes the free iron ions from the surface of the stainless steel. This is what I use on my G40 unit after each brew and it keeps the unit in like new condition.
Traditionally (or more accurately industrially) nitric or citric acid has been used.
If using just an acid the surface needs to be (physically) cleaned prior.
Citric and Nitric acid options require certain times and temperatures to be maintained depending on the grade of acid but for the home user citric acid can often just be used at room temperature. Nitric acid is not recommended for the home user.
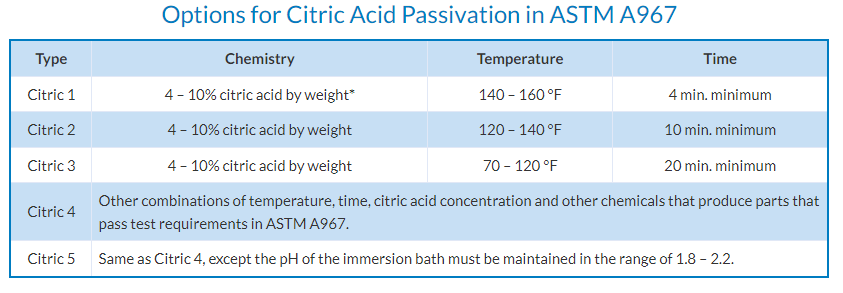

Traditionally (or more accurately industrially) nitric or citric acid has been used.
If using just an acid the surface needs to be (physically) cleaned prior.
Citric and Nitric acid options require certain times and temperatures to be maintained depending on the grade of acid but for the home user citric acid can often just be used at room temperature. Nitric acid is not recommended for the home user.


The deal with BarKeepers friend is that it physically cleans the surface of dirt and physically (and chemically) removes the free iron ions from the surface of the stainless steel. This is what I use on my G40 unit after each brew and it keeps the unit in like new condition.
Traditionally (or more accurately industrially) nitric or citric acid has been used.
If using just an acid the surface needs to be (physically) cleaned prior.
Citric and Nitric acid options require certain times and temperatures to be maintained depending on the grade of acid but for the home user citric acid can often just be used at room temperature. Nitric acid is not recommended for the home user.
View attachment 835083
View attachment 835084
It seems as if they make two versions of BKF, an oxalic acid one (what you buy in the US) and an citric acid one (places overseas). Sadly, that means in the US you would probably still need to use citric acid - you definitely don't want to leave oxalic BKF on your ss any longer than needed for cleaning, i.e. a very few minutes at most (probably don't want to risk the time it might take for passivation, as it can stain the ss). It would be great to have the citric acid version of BKF so you could clean and passivate (better than using oxalic) at the same time as shown in the video.
(edited)
Last edited:
huckdavidson
Well-Known Member
It seems as if they make two versions of BKF, an oxalic acid one (what you buy in the US) and an citric acid one (places overseas). Sadly, that means in the US you would probably still need to use citric acid - you definitely don't want to leave oxalic BKF on your ss any longer than needed for cleaning, i.e. a very few minutes at most (probably don't want to risk the time it might take for passivation, as it can stain the ss). It would be great to have the citric acid version of BKF so you could clean and passivate (better than using oxalic) at the same time as shown in the video.
(edited)
Not according to Bar Keepers Friend themselves:
https://barkeepersfriend.com/cleaning-your-home-brew-supplies/
Has anyone used the liquid bottle version of BKF? I was only able to find that at a hardware store, no powder can.
It's 100% pure anhydrous citric acid.Isn’t concentration of the acid important if you are diluting by weight? I didn’t see that concentration was mention on that Amazon link. Is Citric Acid, that is designed for human consumption, limited to some specific concentration?
Similar threads
- Replies
- 7
- Views
- 744
- Replies
- 13
- Views
- 884
