Sidthesquid
Member
- Joined
- Nov 15, 2016
- Messages
- 13
- Reaction score
- 0
Hi guys is it possible to measure the alcohol content of my homebrew without an original gravity reading? ? I forgot to measure at the start 😣

Yeah you can get close with the #'s and the recipe, but I see you asked is there a way or is it possible to "measure".... No not really. Not without sending it off to a lab.
Cheers
Jay
without an original gravity reading? ? I forgot to measure at the start 😣
Yes you can, if you have the 'correct' gravity reading from a hydrometer and the 'skewed by alcohol' gravity reading from a refractometer. An algorithm for calculating the skew for gravity readings with a refractometer reading can be reversed to give the current ABV, and subsequent OG. There is an error margin of a few gravity points though.
Teach me please. Sounds interesting and VERY worthwhile. I would LOVE to know how to do that without an OG as we get asked ALL the time in the store. I am sure there are PLENTY of people here that can benefit from this info..
Cheers
Jay
Or you could very accurately measure out 10.00 grams of your liquid, heat your sample up to 79degrees celsius (assuming you are at or near sea level), hold it at that temp for about 15-30 minutes agitating it as best as possible, then very accurately weigh your sample again. Subtract your 10.00 gram reading from your final reading and that will tell you how much alcohol you boiled off by weight. Then multiply that number by 1.25 to convert ABW to ABV.
Teach me please. Sounds interesting and VERY worthwhile. I would LOVE to know how to do that without an OG as we get asked ALL the time in the store. I am sure there are PLENTY of people here that can benefit from this info..
Cheers
Jay
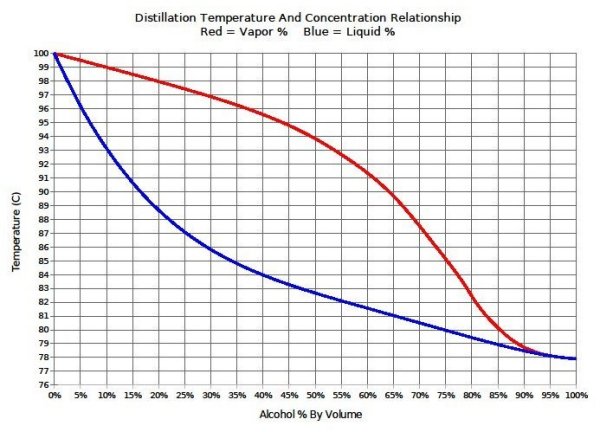
A good idea, but this wouldn't work. Although the boiling point of ethanol is 78C, a mixture containing ethanol and water won't boil until much warmer than 78C and comes off as a mixture, not as pure ethanol. So, at 79C you'd get very little evaporation and what did evaporate would be well below 100% ethanol. With your method (at a warmer temp), you'd need to also catch the distillate and measure the alcohol content with an alcoholmeter, but then you'd be distilling which is illegal in most places.
Enter your email address to join: