Disclaimer: I am diving head first in water chemistry and doing my best to not be overwhelmed. Please excuse any newbie mistakes.
Below are screenshots of all relevant info placed into Bru'n Water. I hope that I am inputting everything correctly for a 100% RO water base. If anyone can please let me know if I am on the right track that would be great.
Comments on the above photos
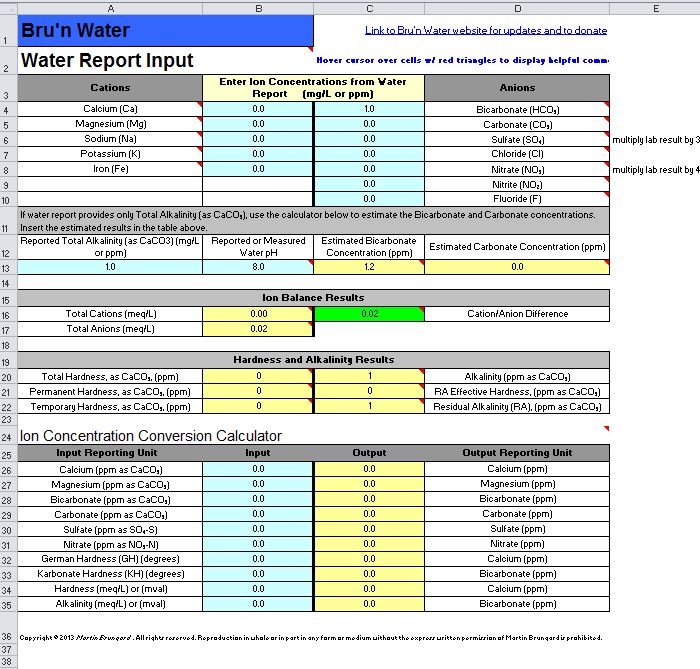
1. Water Report Input - Did Not Touch
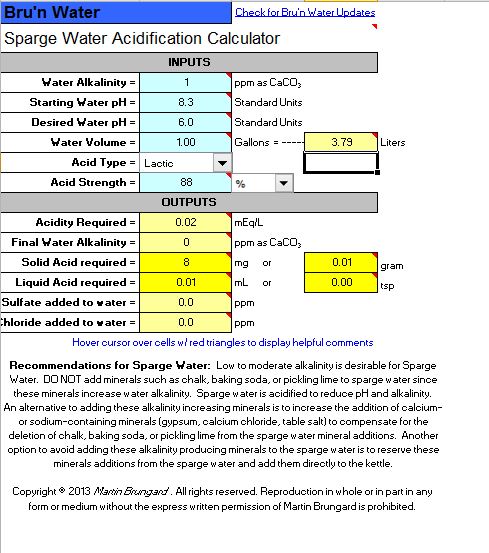
2. Sparge Acidification - Did Not Touch
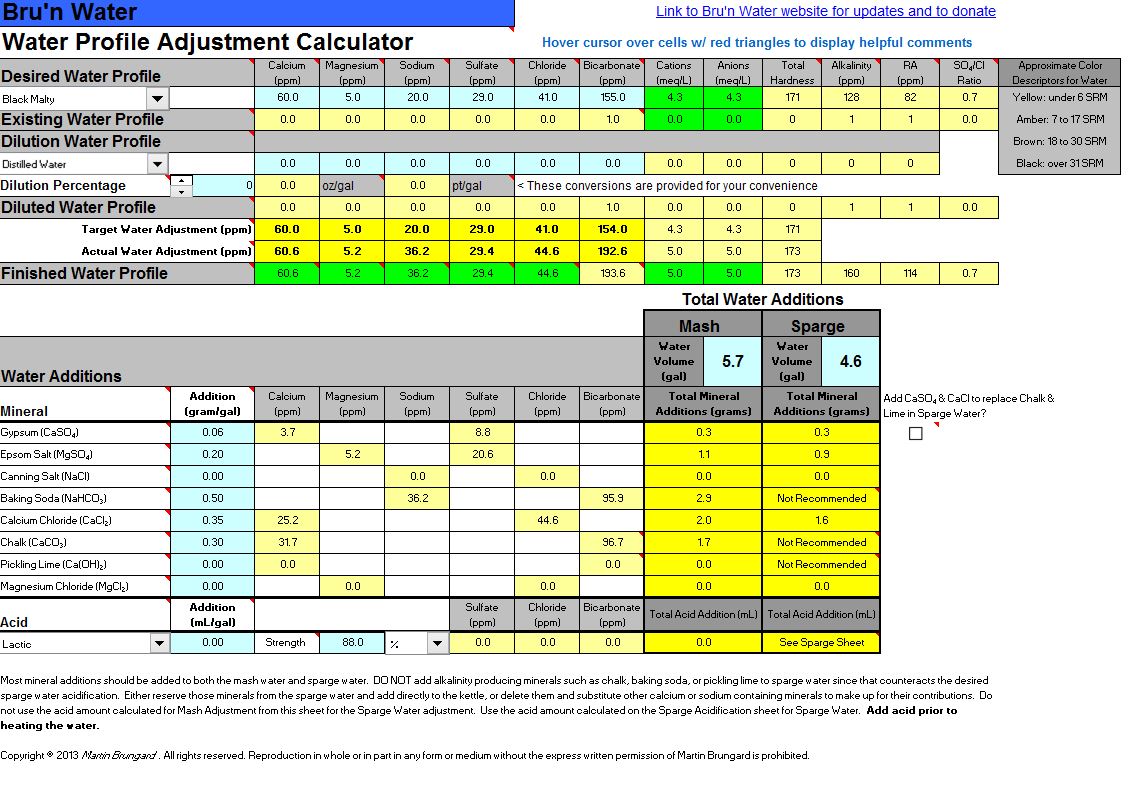
3. Water Adjustment - According to the Mash Acidification tab, I had to raise my alkalinity to get the PH in range. I am in Canada and cannot seem to find Pickling Lime. I have found Kalkwasser on an online shop but do not know if this is appropriate for brewing. I have shown chalk additions even though I have read some strong opinions to not use it due to the difficulty in dissolving it in water. This article from Braukaiser lays out a way to dissolve chalk with the help of CO2. Any guidance on what to do here would be great.
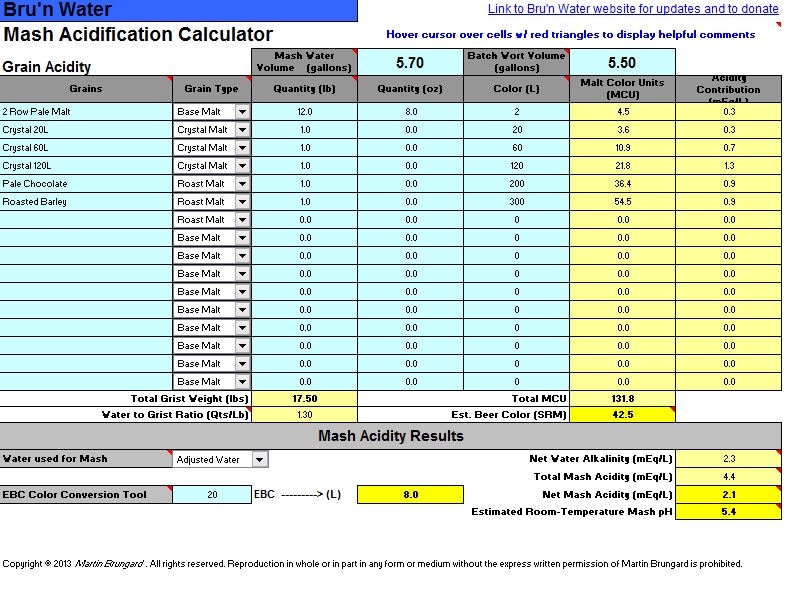
4. Mash Acidification - Big grain bill, I know. This is for a Tart Cherry Stout I am making, EST OG 1.069 (ABV 7.6%) based on my system average efficiency of 68%.
If any further information is required from me please let me know. If anyone can provide me with some feedback on whether I am on the right track, I would appreciate it greatly.
Edit: Needed to fix 4th image.
Below are screenshots of all relevant info placed into Bru'n Water. I hope that I am inputting everything correctly for a 100% RO water base. If anyone can please let me know if I am on the right track that would be great.




Comments on the above photos
1. Water Report Input - Did Not Touch
2. Sparge Acidification - Did Not Touch
3. Water Adjustment - According to the Mash Acidification tab, I had to raise my alkalinity to get the PH in range. I am in Canada and cannot seem to find Pickling Lime. I have found Kalkwasser on an online shop but do not know if this is appropriate for brewing. I have shown chalk additions even though I have read some strong opinions to not use it due to the difficulty in dissolving it in water. This article from Braukaiser lays out a way to dissolve chalk with the help of CO2. Any guidance on what to do here would be great.
4. Mash Acidification - Big grain bill, I know. This is for a Tart Cherry Stout I am making, EST OG 1.069 (ABV 7.6%) based on my system average efficiency of 68%.
If any further information is required from me please let me know. If anyone can provide me with some feedback on whether I am on the right track, I would appreciate it greatly.
Edit: Needed to fix 4th image.

