Munich (Dark Lager)
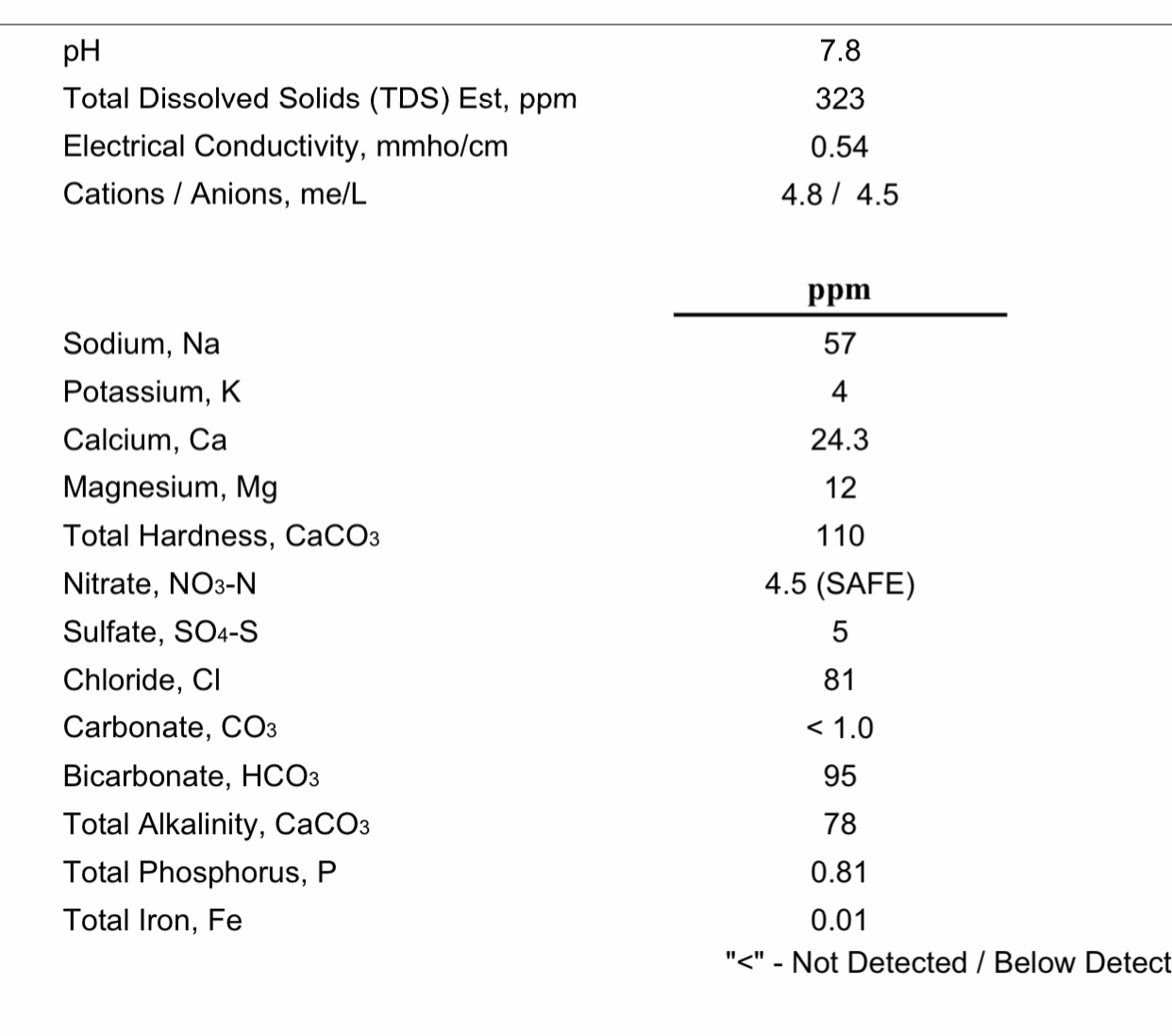
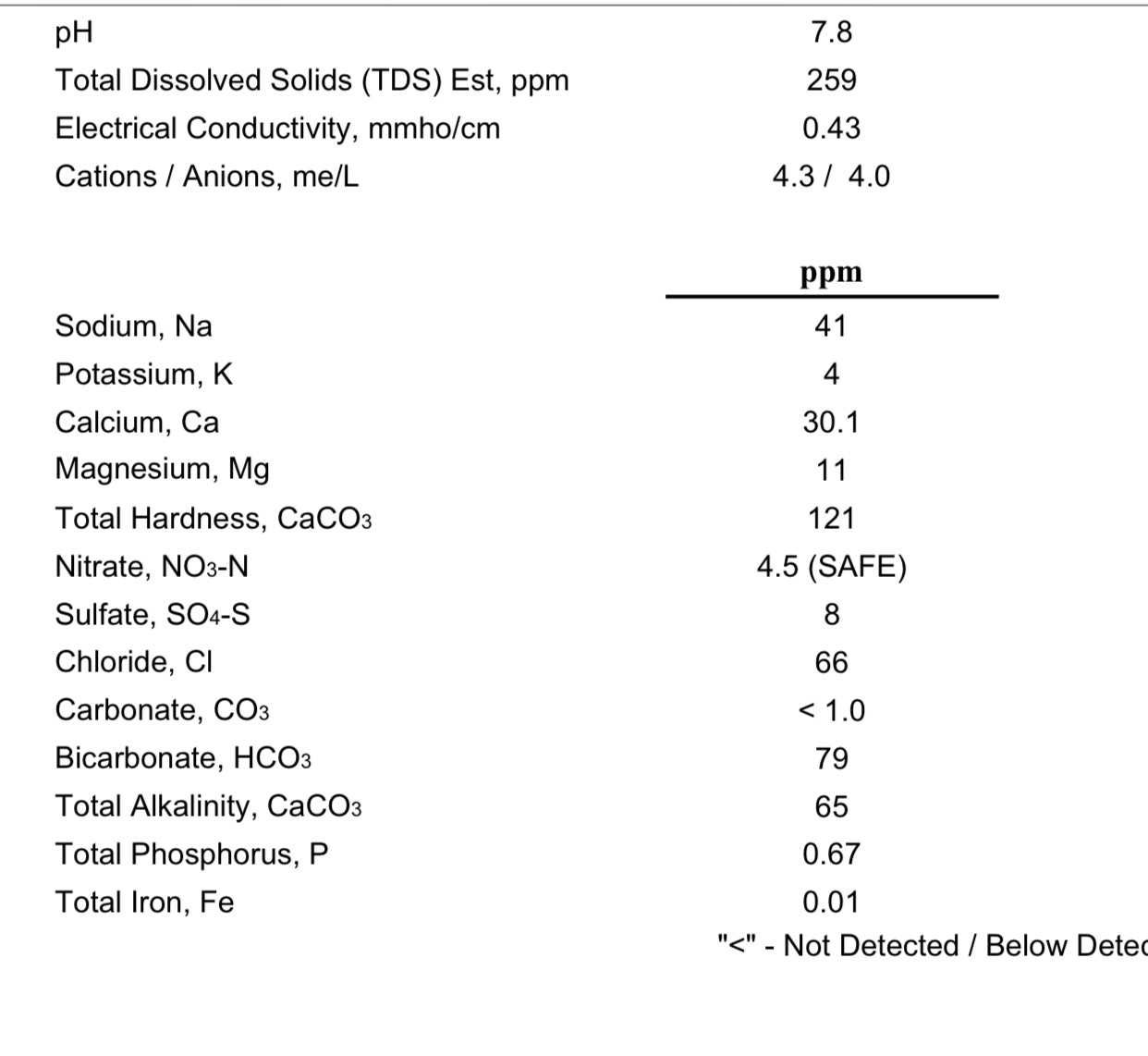
How the hell are you supposed to adjust water to 82 ca and 20 mg when you can’t use any CaCl2, CaSo4, or MgSO4 to keep 2 Cl and 16 SO4? Ridiculous.
| Ca2+ | Mg2+ | Na+ | Cl- | SO42- | HCO3- |
|---|---|---|---|---|---|
| Ion Profile in ppm | |||||
| 82 | 20 | 4 | 2 | 16 | 320 |
How the hell are you supposed to adjust water to 82 ca and 20 mg when you can’t use any CaCl2, CaSo4, or MgSO4 to keep 2 Cl and 16 SO4? Ridiculous.