hlmbrwng
Well-Known Member
I was motivated to purchase a relatively inexpensive microscope having x1000 magnification so I can start looking at microbes after my brews, to look at microbes of commercial beers, and to perform cell counts.
The last couple of batches have given off a strange plasticy smell. Actually, the last batch really did, and I think the fermentation chamber just still smells like it. Not sure that the new batch is infected.
I transfered the beer to secondary so that I could do a second dry hopping of this beer. It is a hefeweizen using Wyeast 3068. I was waiting to receive immersion oil in the mail, so the mostly empty carboy sat for a day with its opening covered with plastic wrap (a couple of hours went by before I thought to cover it).
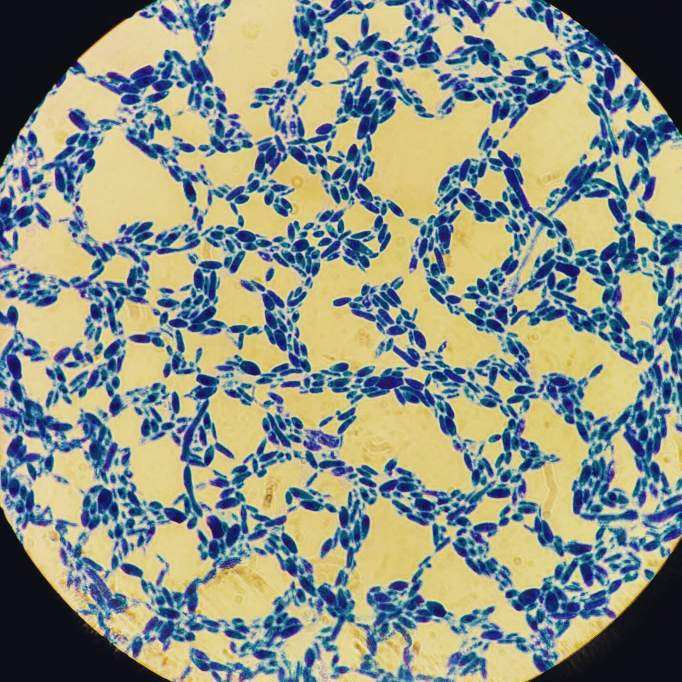
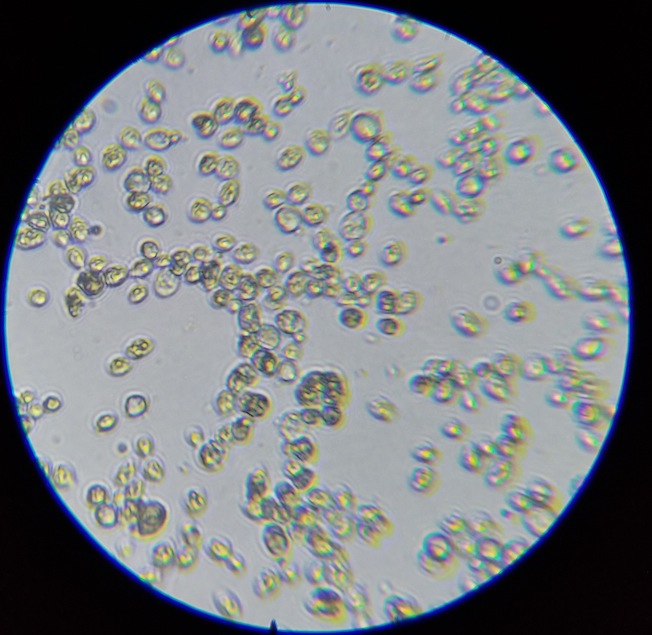
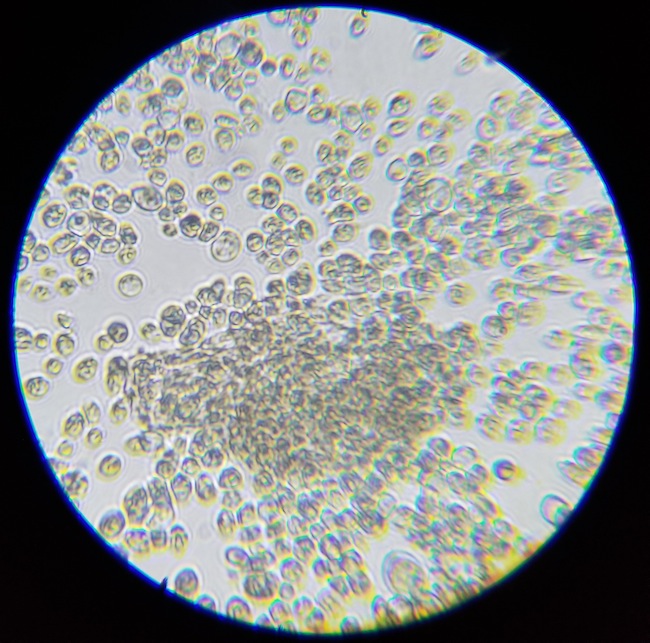
The mesh bag containing the first round of dry-hop pellets was still in the carboy, so there was A LOT of hop material. I added some water, swished it all around, dump a bunch in a glass and let it sit for an hour. I pulled some beer from the top and placed it on a slide to view. I did not diluate the liquid to any specific degree. Here are some images. Thoughts on what we are seeing would be appreciated. I'm having a hard time knowing how to tell sacc from some brett strains, since they can look similar. Also, are we seeing hop material? Autolysis?
1) This is pretty much what most of the slide looked like
2) Same
3) Mostly the same
4) Some different looking "cells"? Autolysis? Hop material or oil?
5) What is in the center, hop material?



The last couple of batches have given off a strange plasticy smell. Actually, the last batch really did, and I think the fermentation chamber just still smells like it. Not sure that the new batch is infected.
I transfered the beer to secondary so that I could do a second dry hopping of this beer. It is a hefeweizen using Wyeast 3068. I was waiting to receive immersion oil in the mail, so the mostly empty carboy sat for a day with its opening covered with plastic wrap (a couple of hours went by before I thought to cover it).
The mesh bag containing the first round of dry-hop pellets was still in the carboy, so there was A LOT of hop material. I added some water, swished it all around, dump a bunch in a glass and let it sit for an hour. I pulled some beer from the top and placed it on a slide to view. I did not diluate the liquid to any specific degree. Here are some images. Thoughts on what we are seeing would be appreciated. I'm having a hard time knowing how to tell sacc from some brett strains, since they can look similar. Also, are we seeing hop material? Autolysis?
1) This is pretty much what most of the slide looked like
2) Same
3) Mostly the same
4) Some different looking "cells"? Autolysis? Hop material or oil?
5) What is in the center, hop material?