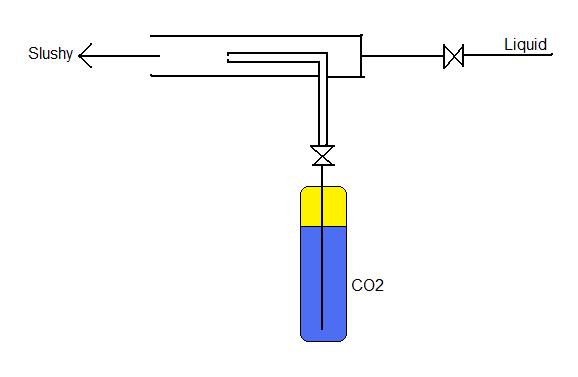
From what you have kind of drawn there it looks like you want to mix the co2 and liquid before the mixing container? If so I woul think that if the plan is to use the liquid CO2 to cool the liquid, what you have drawn there will not work. Firstly you state you have a regulator (this term would usually be applied to a pressure regulator in case like this), what you want is a metering valve to adjust the flow but to make sure you do not get a large pressure drop accross this and evaporate the liquid CO2 at this point you will need an oriface (read: small hole) at the point of injection - this was mentioed earlier.
Second you have the liquid coming into the mixing point, unless you can get the liquid up to the pressure of liquid CO2 at room temp (870ish PSI) all that will happen is you will push the liquid back with the CO2. I would think a venturi mixing could possibly work in this case.
Are you going forward with a prototype to develop into a comercial product or is this just some DIY hack you want to do.
If it is the first you really need to engage an engineer with experience in pressure equipment and food & beverage applications. When you do make sure you get them to sign a properly drafted non disclosure statement (for that you will probably also need to see a lawyer

)
If it is the second, why they hell can't you just tell us what you are planning on doing so we can actually help

Lastly if you are trying to make a slushy machine with CO2, when apparently they already exist with N2 (I think you said they were available?) stop and think if making it out of CO2 makes actual sense - CO2 is more expensive to produce and harder to handle use.


 That's also why you want to keep your co2 tank upright when connected to your regulator. If it were turned upside down, you'd end up with liquid co2 in your regulator, and then in your keg. Not good.
That's also why you want to keep your co2 tank upright when connected to your regulator. If it were turned upside down, you'd end up with liquid co2 in your regulator, and then in your keg. Not good.