loveofrose
Well-Known Member
I’ve been quiet as of late because I got lost in the yeast lab I built. In the past, I’ve isolated yeast from honey, but this time I’m taking it quite a bit further. I’m purifying everything to a single strain of yeast and banking it forever. Eventually, I want to start my own Meadery with the isolated strain(s).
The goal is to have my own personal “mead yeast” that actually comes from honey. The catch: this yeast has to be stellar. It has to give body, ferment clean and enhance aroma. While I’m wishing, I would like a low and high ABV yeast as well.
I’m trying a more hardcore approach this time and though I would document my findings here for those of you wishing to embark on a epic journey such as this. I’ve refined my technique to make things less complicated (still complicated though), streamlined and efficient.
Growing Microbes
First, you need honey. In my case, I’ve amassed 14 different varietal honeys. I’m attempting to isolate yeast from all of them. I designate them A-N. Then, I do the following:
1. Add 1 ml of honey plus 4 ml of water to a 15 ml tube using aseptic technique. If you don’t know aseptic technique involving a flame, flame loop and petri plates, YouTube is your friend.
2. Cap and vortex tube until fully mixed.
3. Take a gravity reading to determine SG with a refractometer.
4. Adjust SG with water to fall into the 1.070-1.090 range.
5. Cap the tubes and incubate at 70 F for 1-4 weeks. Some grow quickly, some slow and some not at all.
6. Once you see a small pellet in the bottom of the tube, it’s time to go to the next section.
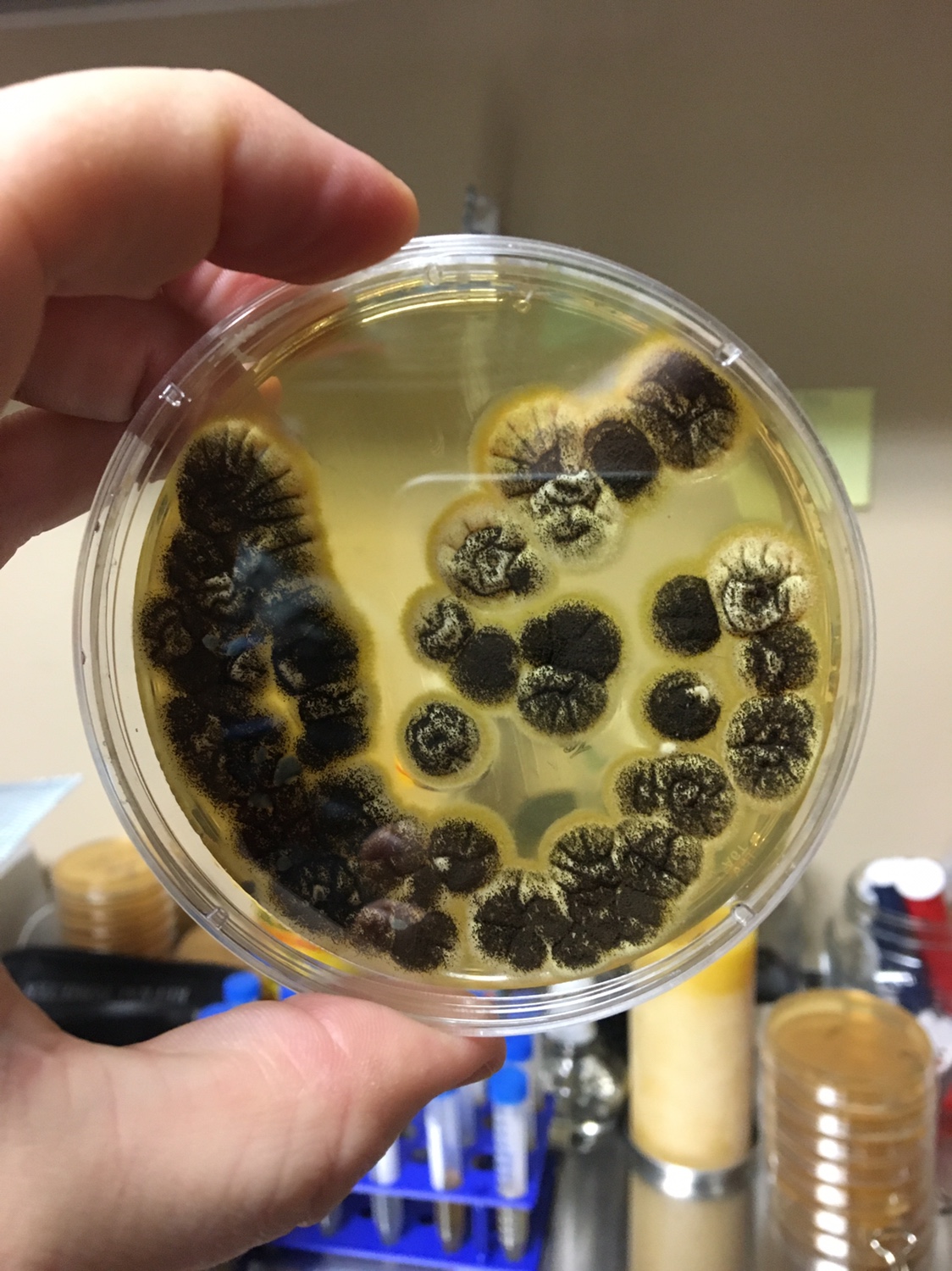
Pro tip: Document the smell of the mead at this point. You want to compare it to the pure strain later. Don’t smell anything with black mold however.
Microbe Isolation
1. Begin by analyzing all the tubes for fuzzy growth on top of the mead. If you see a substantial amount, it is best to use a sterilized flame loop to grab and discard this. It is filamentous fungi and not the yeast you are after. It will also contaminate what you are going for.
2. Get your flame loop red hot and plunge it to the bottom of the tube. Try to swirl the pellet gently. Then remove the loop without touching the sides.
Note: Since the loop is red hot when it hits the surface, you reduce contamination from the floaters. It’s cooled by the time you hit the pellet.
3. Streak using the quadrant method (again, YouTube) on both YPD plates and MEA + 0.01% chloramphenicol. You can just do YPD if you want, but I’m trying both.
4. Incubate plates upside down at 70 F until you see growth. This can be a day or a month.
5. Once you see growth, it will likely be a mixture of yeast, filamentous fungi, Staphylococcus, Pediococcus, and other nasties. The goal now is to isolate only the yeast.
Pure Yeast Isolation
1. First, you need to know what a yeast looks like on a Petri dish. Generally, the colonies are white to off white, round, shiny, smooth colonies that appear to grow on top of the agar. Avoid Fuzzy, black (mold), green (penicillium/Psuedomonas), red (Serratia), yellow (Staphylococcus) and growing into the agar (filamentous fungi) type colonies.
2. Find a well isolated colony and restreak it on a new plate.
3. Incubate at 70 F for 1-2 days.
4. Check to see if the plate looks like all one type of colony. If not, repeat steps 2-4 until it breeds true. This can sometimes happen on the first restreak or it may take ten.
5. You now have a single strain of yeast. Now for the real test. Is it any good?
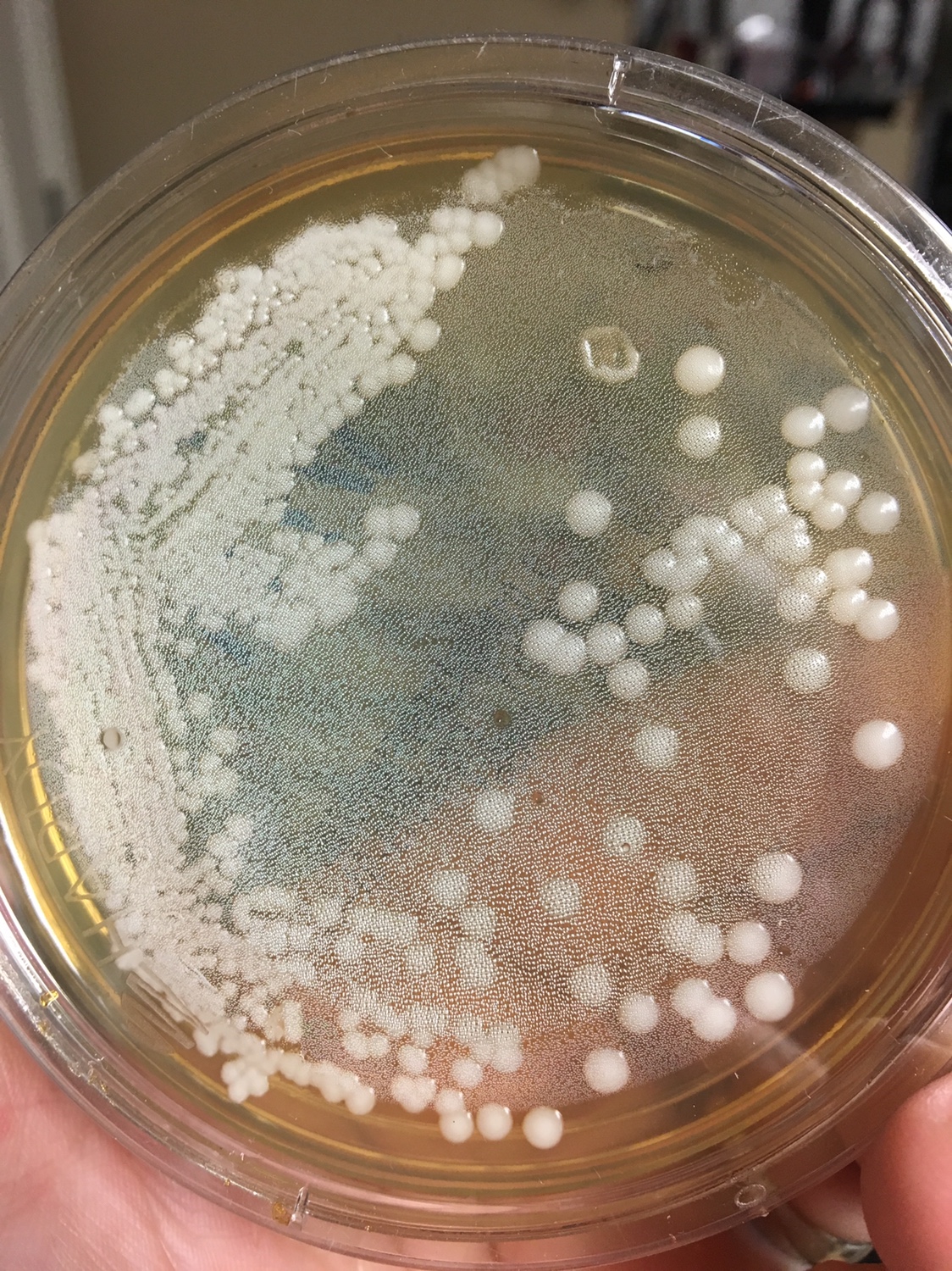
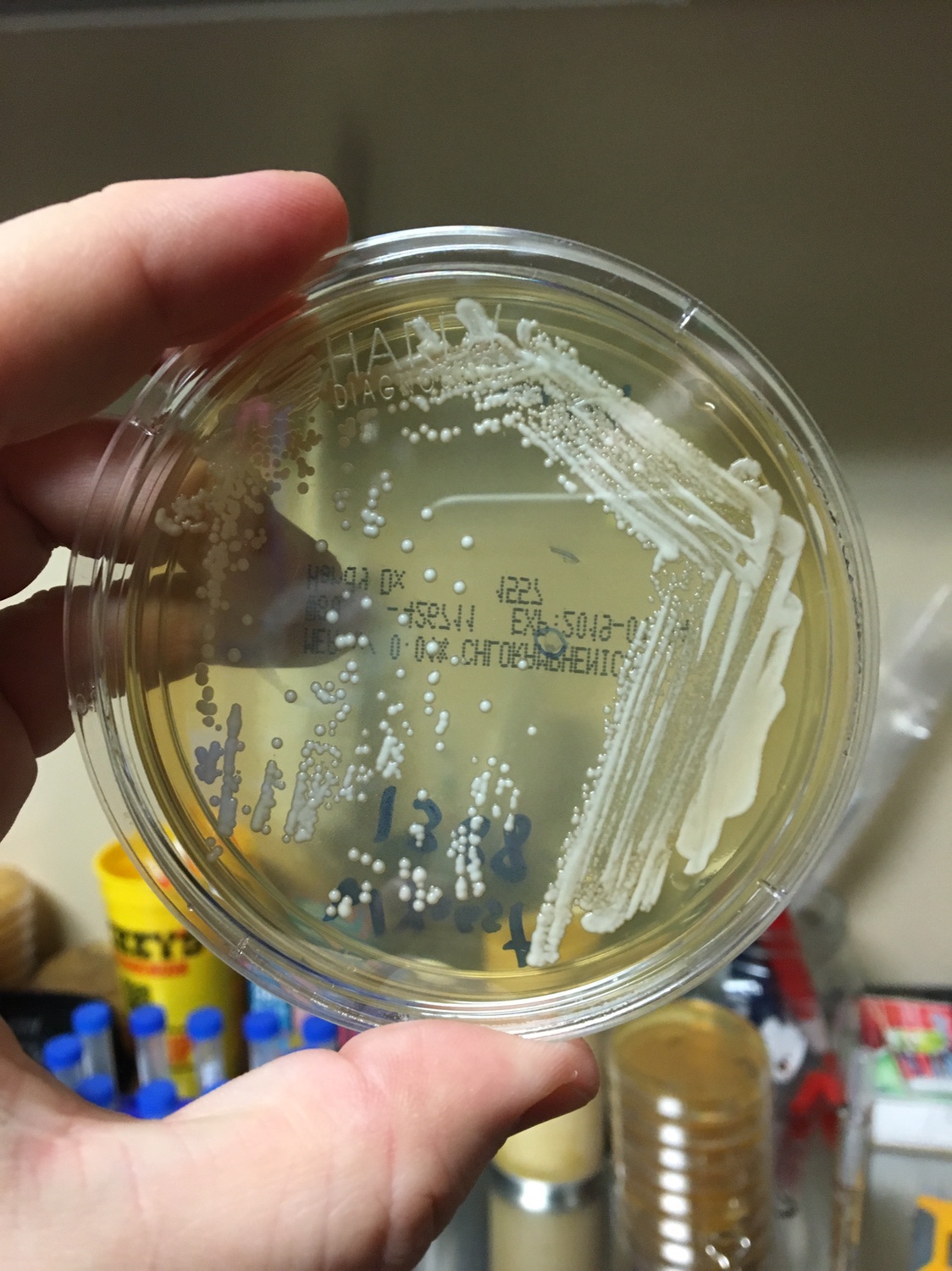
Yeast Example 1
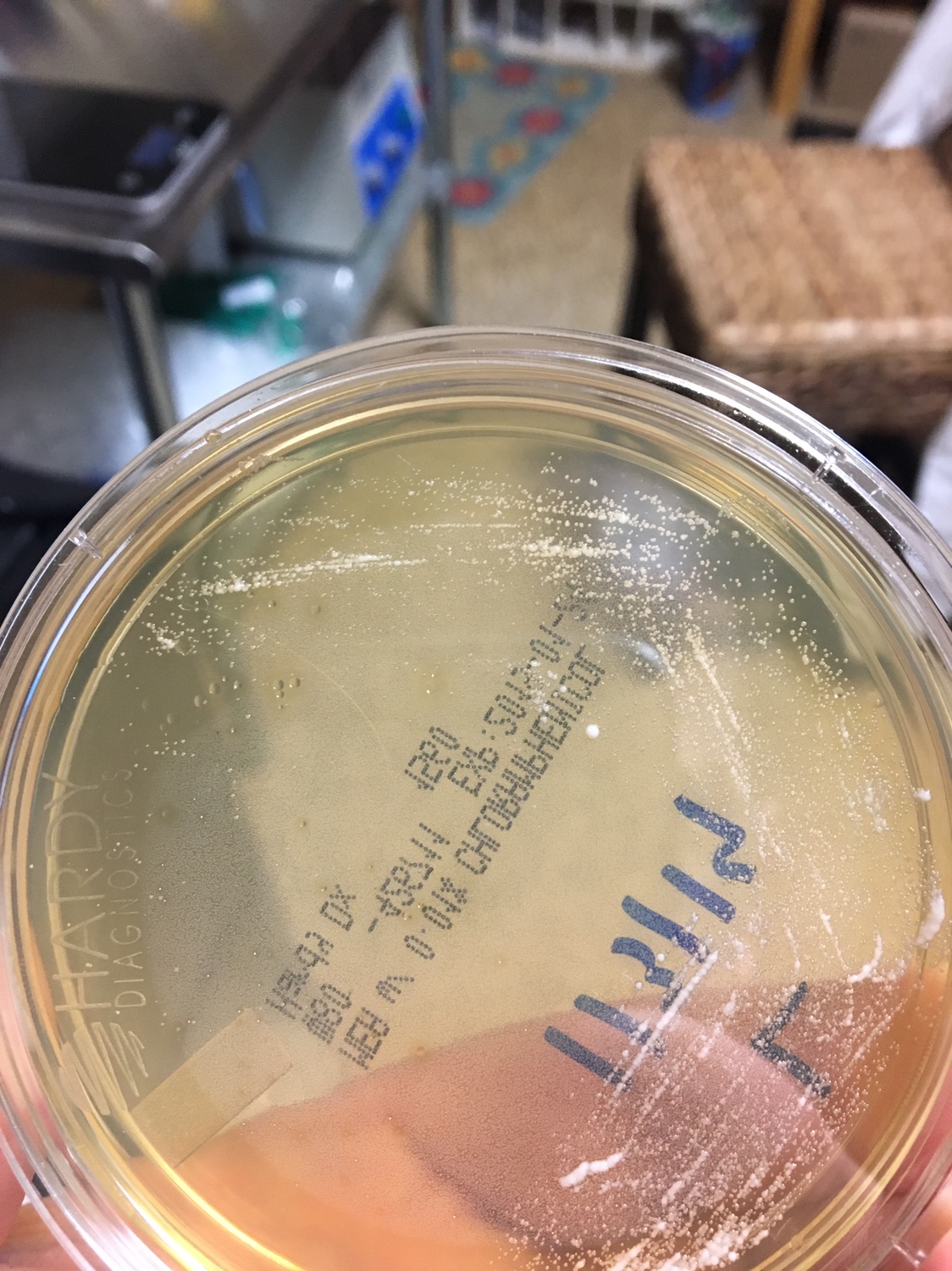
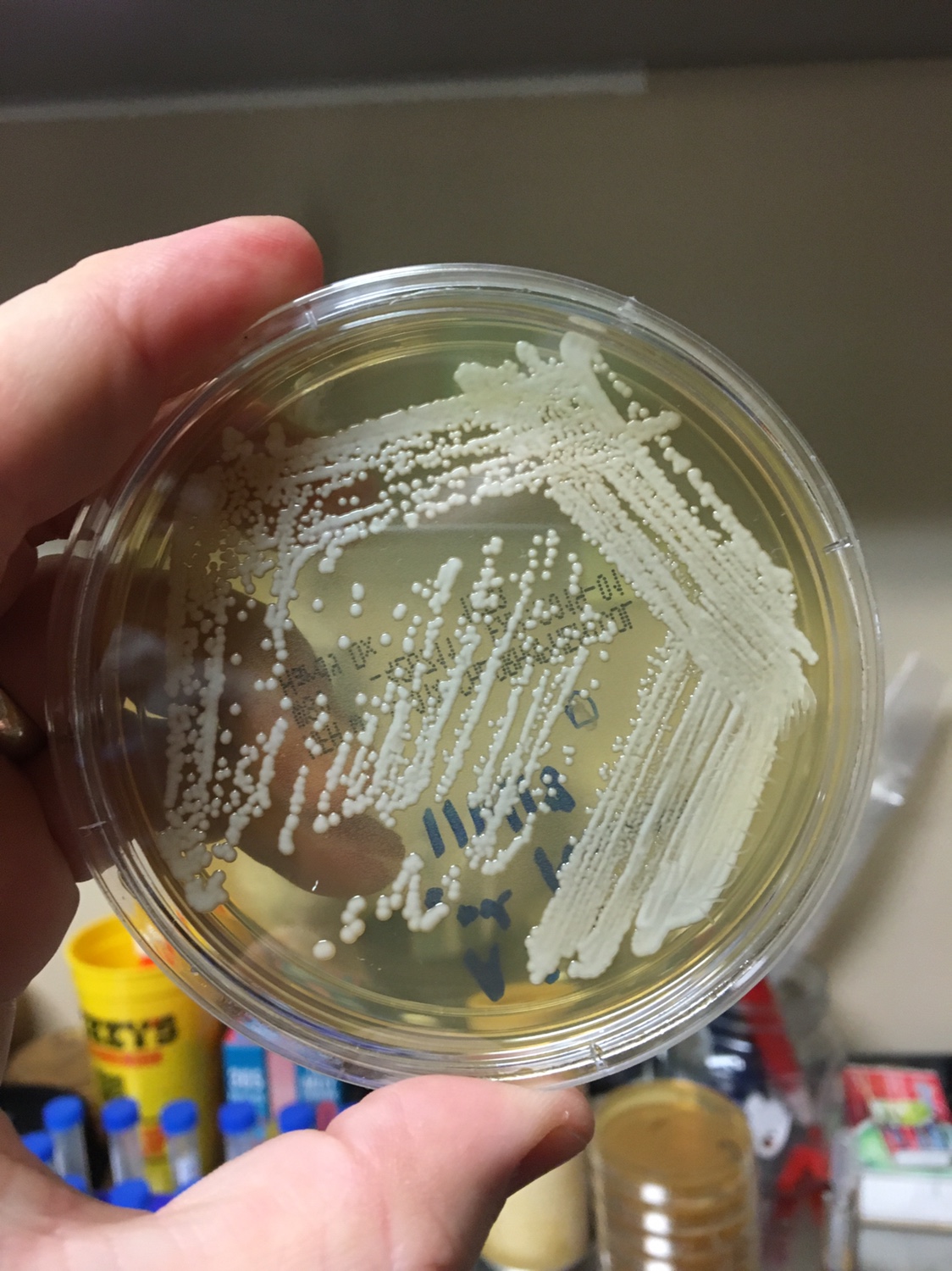
Yeast Example 2
Mead Yeast Testing
Now the fun part. What do your new isolates taste like in mead? First, we start with an 8% ABV mead as some yeast have trouble past this, then we test how high it can go. Since I’m testing so many, I’ve developed a micro scale test as follows:
1. To a 50 ml tube, add 40 mg of Fermaid O, 5 ml honey, 35 ml spring water.
2. Vortex until homogeneous and take a refractometer reading.
3. Using a sterilized flame loop, add a loop full of your yeast isolate.
4. Incubate at 70 F until the mead goes dry (<1.000) or the yeast gives up. Sometime the yeast is unable to ferment this gravity, so you will have to dilute with water. There is a lot of variability here so expect surprises.
5. Since this is done in a 50 ml tube, we centrifuge it at 6000 rpm for 15 minutes to pellet the yeast.
6. Decant the mead into an evaluation glass for testing.

Micro Mead Test
At this point, it’s either good, bad, or ugly. Winning yeast that are pleasing go to the next step.
Mead Yeast Characterization
If you are establishing a a new house yeast, you need to at least know a few basics such as the following:
1. ABV tolerance: Inoculate must at 1.080, 1.100, 1.120, and 1.140. Go higher or lower as needed.
2. Temperature: Try 65, 70, 75 F as a start and taste the meads to determine the best. Refine as needed once you have a ballpark range.
3. Nutrient Requirements: Try low, medium and high level of Fermaid O/ YAN and taste test the resulting mead.
Many more things can be characterized, but this is the core to start with.
So what success rate to expect? Out of 14 honeys, I got 4 plates with true yeast, 5 plates of filamentous fungi, 1 plate of black mold and 4 blank plates. Of those 4 yeast, perhaps 1 will be good enough to use. The testing is still ongoing. I’ll keep you posted here!
The goal is to have my own personal “mead yeast” that actually comes from honey. The catch: this yeast has to be stellar. It has to give body, ferment clean and enhance aroma. While I’m wishing, I would like a low and high ABV yeast as well.
I’m trying a more hardcore approach this time and though I would document my findings here for those of you wishing to embark on a epic journey such as this. I’ve refined my technique to make things less complicated (still complicated though), streamlined and efficient.
Growing Microbes
First, you need honey. In my case, I’ve amassed 14 different varietal honeys. I’m attempting to isolate yeast from all of them. I designate them A-N. Then, I do the following:
1. Add 1 ml of honey plus 4 ml of water to a 15 ml tube using aseptic technique. If you don’t know aseptic technique involving a flame, flame loop and petri plates, YouTube is your friend.
2. Cap and vortex tube until fully mixed.
3. Take a gravity reading to determine SG with a refractometer.
4. Adjust SG with water to fall into the 1.070-1.090 range.
5. Cap the tubes and incubate at 70 F for 1-4 weeks. Some grow quickly, some slow and some not at all.
6. Once you see a small pellet in the bottom of the tube, it’s time to go to the next section.
Pro tip: Document the smell of the mead at this point. You want to compare it to the pure strain later. Don’t smell anything with black mold however.
Microbe Isolation
1. Begin by analyzing all the tubes for fuzzy growth on top of the mead. If you see a substantial amount, it is best to use a sterilized flame loop to grab and discard this. It is filamentous fungi and not the yeast you are after. It will also contaminate what you are going for.
2. Get your flame loop red hot and plunge it to the bottom of the tube. Try to swirl the pellet gently. Then remove the loop without touching the sides.
Note: Since the loop is red hot when it hits the surface, you reduce contamination from the floaters. It’s cooled by the time you hit the pellet.
3. Streak using the quadrant method (again, YouTube) on both YPD plates and MEA + 0.01% chloramphenicol. You can just do YPD if you want, but I’m trying both.
4. Incubate plates upside down at 70 F until you see growth. This can be a day or a month.
5. Once you see growth, it will likely be a mixture of yeast, filamentous fungi, Staphylococcus, Pediococcus, and other nasties. The goal now is to isolate only the yeast.
Pure Yeast Isolation
1. First, you need to know what a yeast looks like on a Petri dish. Generally, the colonies are white to off white, round, shiny, smooth colonies that appear to grow on top of the agar. Avoid Fuzzy, black (mold), green (penicillium/Psuedomonas), red (Serratia), yellow (Staphylococcus) and growing into the agar (filamentous fungi) type colonies.
2. Find a well isolated colony and restreak it on a new plate.
3. Incubate at 70 F for 1-2 days.
4. Check to see if the plate looks like all one type of colony. If not, repeat steps 2-4 until it breeds true. This can sometimes happen on the first restreak or it may take ten.
5. You now have a single strain of yeast. Now for the real test. Is it any good?

Yeast Example 1

Yeast Example 2
Mead Yeast Testing
Now the fun part. What do your new isolates taste like in mead? First, we start with an 8% ABV mead as some yeast have trouble past this, then we test how high it can go. Since I’m testing so many, I’ve developed a micro scale test as follows:
1. To a 50 ml tube, add 40 mg of Fermaid O, 5 ml honey, 35 ml spring water.
2. Vortex until homogeneous and take a refractometer reading.
3. Using a sterilized flame loop, add a loop full of your yeast isolate.
4. Incubate at 70 F until the mead goes dry (<1.000) or the yeast gives up. Sometime the yeast is unable to ferment this gravity, so you will have to dilute with water. There is a lot of variability here so expect surprises.
5. Since this is done in a 50 ml tube, we centrifuge it at 6000 rpm for 15 minutes to pellet the yeast.
6. Decant the mead into an evaluation glass for testing.

Micro Mead Test
At this point, it’s either good, bad, or ugly. Winning yeast that are pleasing go to the next step.
Mead Yeast Characterization
If you are establishing a a new house yeast, you need to at least know a few basics such as the following:
1. ABV tolerance: Inoculate must at 1.080, 1.100, 1.120, and 1.140. Go higher or lower as needed.
2. Temperature: Try 65, 70, 75 F as a start and taste the meads to determine the best. Refine as needed once you have a ballpark range.
3. Nutrient Requirements: Try low, medium and high level of Fermaid O/ YAN and taste test the resulting mead.
Many more things can be characterized, but this is the core to start with.
So what success rate to expect? Out of 14 honeys, I got 4 plates with true yeast, 5 plates of filamentous fungi, 1 plate of black mold and 4 blank plates. Of those 4 yeast, perhaps 1 will be good enough to use. The testing is still ongoing. I’ll keep you posted here!