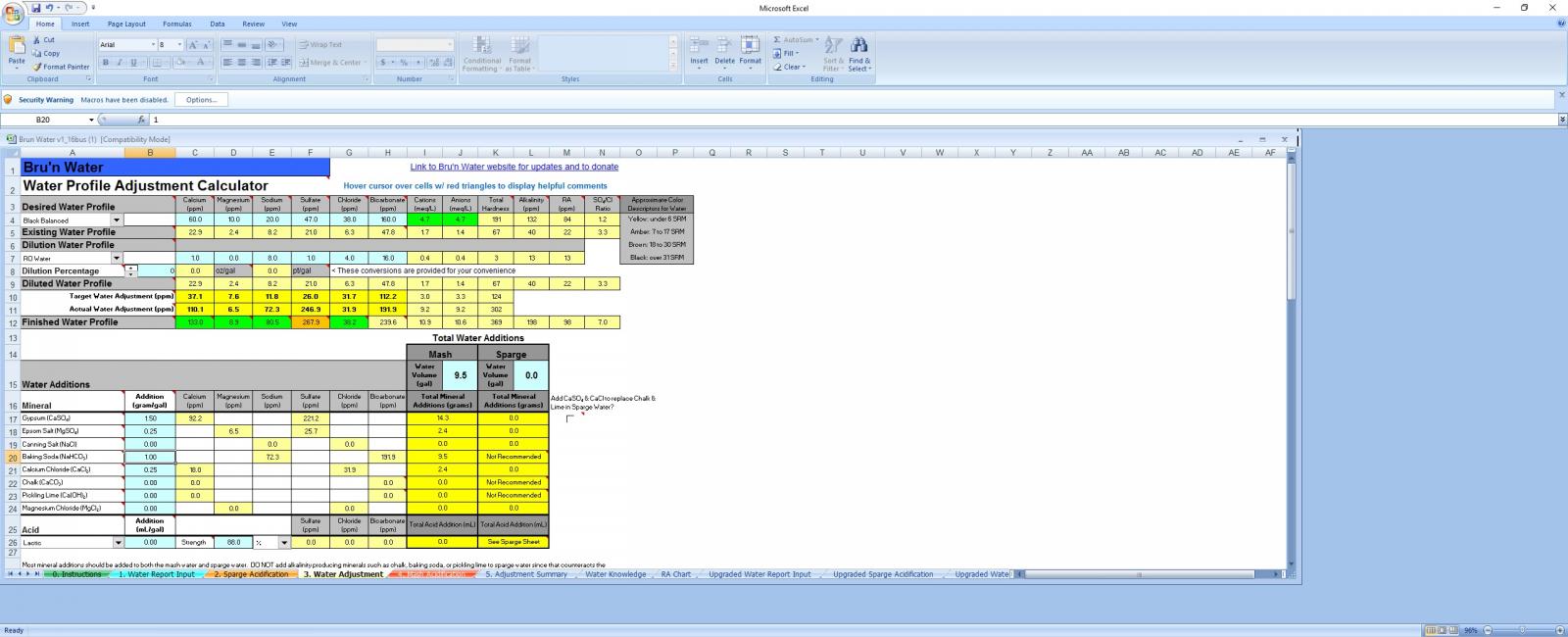
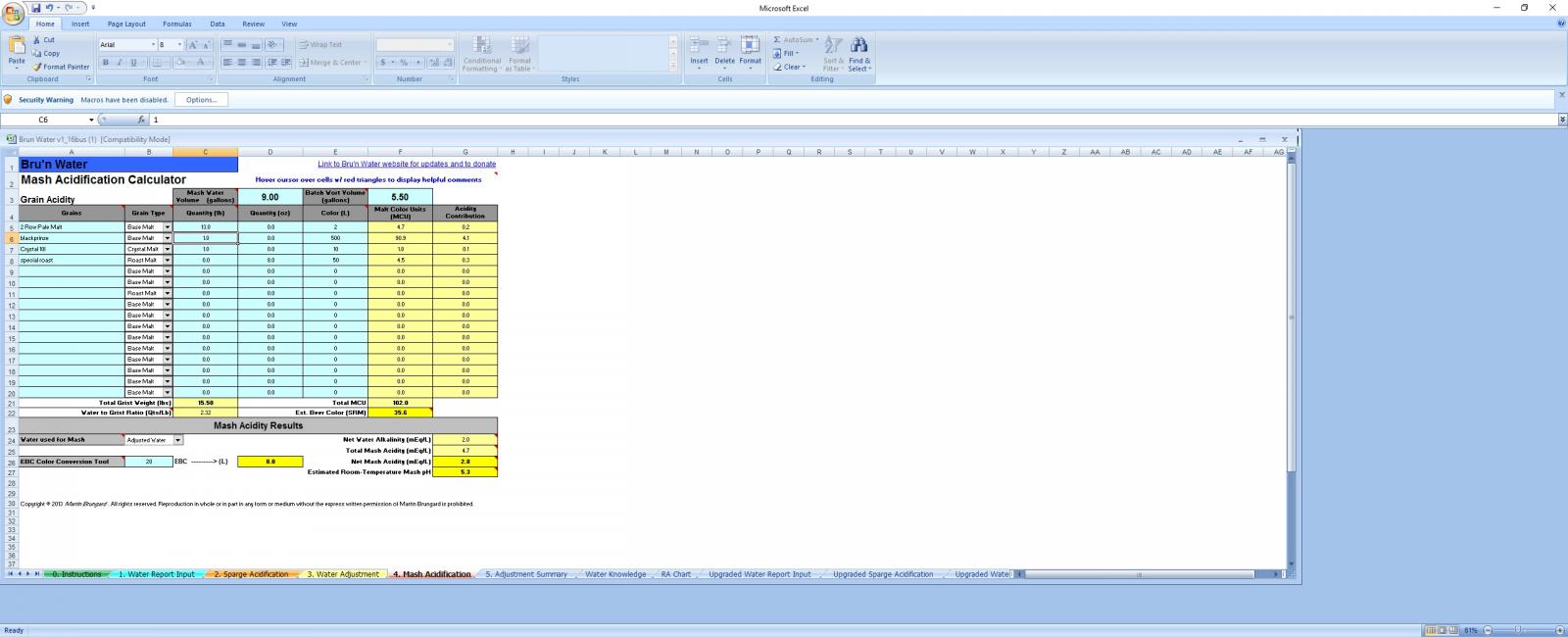
Hello
beginner with water chem here. Full boil BIAB
my plan is a cipa with
1) 13# 2 row
2) 1# crystal 10L
3) 1# blackprinze 500L
4) 0.5 special roast 50L
i have tried to use brunwater and ez with my water and with 100% RO but i keep getting a requirement to add alkalinity. When i add baking soda it brings the Na way high and im having to use about 10gm of baking soda to the pH up to about 5.4
im guessing its the blackprinze driving the pH down
is that ok?
without the baking soda and alot of it i get a pH of 4.7 at room temp.
Im trying to avoid high sodium levels in the 80ppm range, but is dumping 10+gms of baking soda to alkali the strike water ok for this style of beer?? can i get away with a high Na level??
thx for any advice. im trying to figure out how to submit a screenshot of excel bc im an idiot...
beginner with water chem here. Full boil BIAB
my plan is a cipa with
1) 13# 2 row
2) 1# crystal 10L
3) 1# blackprinze 500L
4) 0.5 special roast 50L
i have tried to use brunwater and ez with my water and with 100% RO but i keep getting a requirement to add alkalinity. When i add baking soda it brings the Na way high and im having to use about 10gm of baking soda to the pH up to about 5.4
im guessing its the blackprinze driving the pH down
is that ok?
without the baking soda and alot of it i get a pH of 4.7 at room temp.
Im trying to avoid high sodium levels in the 80ppm range, but is dumping 10+gms of baking soda to alkali the strike water ok for this style of beer?? can i get away with a high Na level??
thx for any advice. im trying to figure out how to submit a screenshot of excel bc im an idiot...