WHAT CAN BE DONE TO PREVENT ILLNESS?
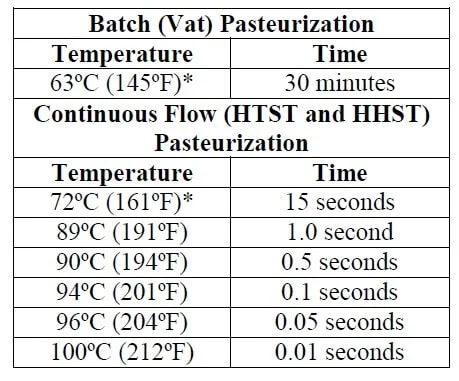
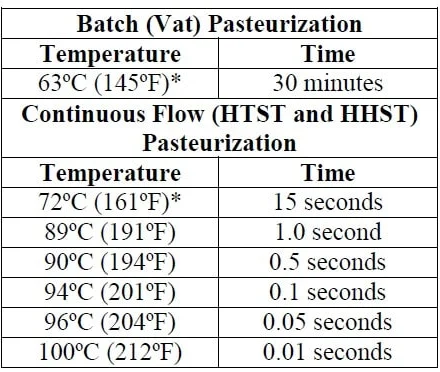
Primary growth-limiting factors for C. botulinum include environmental temperature above 250°F (121°C) or below 39°F (4°C); high acidity (pH <4.6); low water activity (lack of available moisture); food preservatives such as nitrite, sorbic acid, phenolic antioxidants, polyphosphates, and ascorbates; a low redox potential (absence of oxygen); and competing microorganisms (Sobel et al. 2004). To be safe, the FDA 2013 Food Code recommendation is that food be kept out of the 'Danger Zone'. Thus, for safety against this pathogen and others, store food items below 41°F (5°C) and hold hot food above 135°F (57°C) (FDA 2013). Due to their low water activity, dehydrated foods and foods high in salt and/or sugar do not support growth of C. botulinum. Some strains of C. botulinum can be mesophilic, with an ideal growth temperature between 68°F–113°F (20°C–45°C), whereas others are psychotropic, with ideal growth between 38°F–60°F (3°C–20°C). Proper cooking and handling of food is important for the elimination of C. botulinum, because growth is possible at a wide range of environmental temperatures. Although C. botulinum typically will not grow in environments of pH <4.6, food proteins, such as those in soy and beef, can have a protective effect on the bacteria by providing localized areas or pockets of high pH, thus allowing for growth in high-acid foods (Wong et al. 1988).