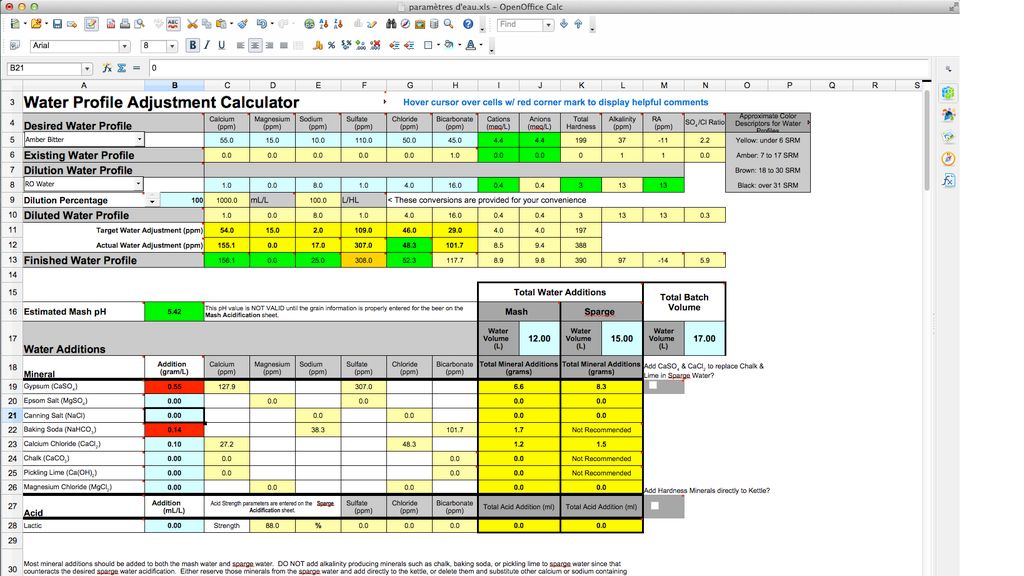
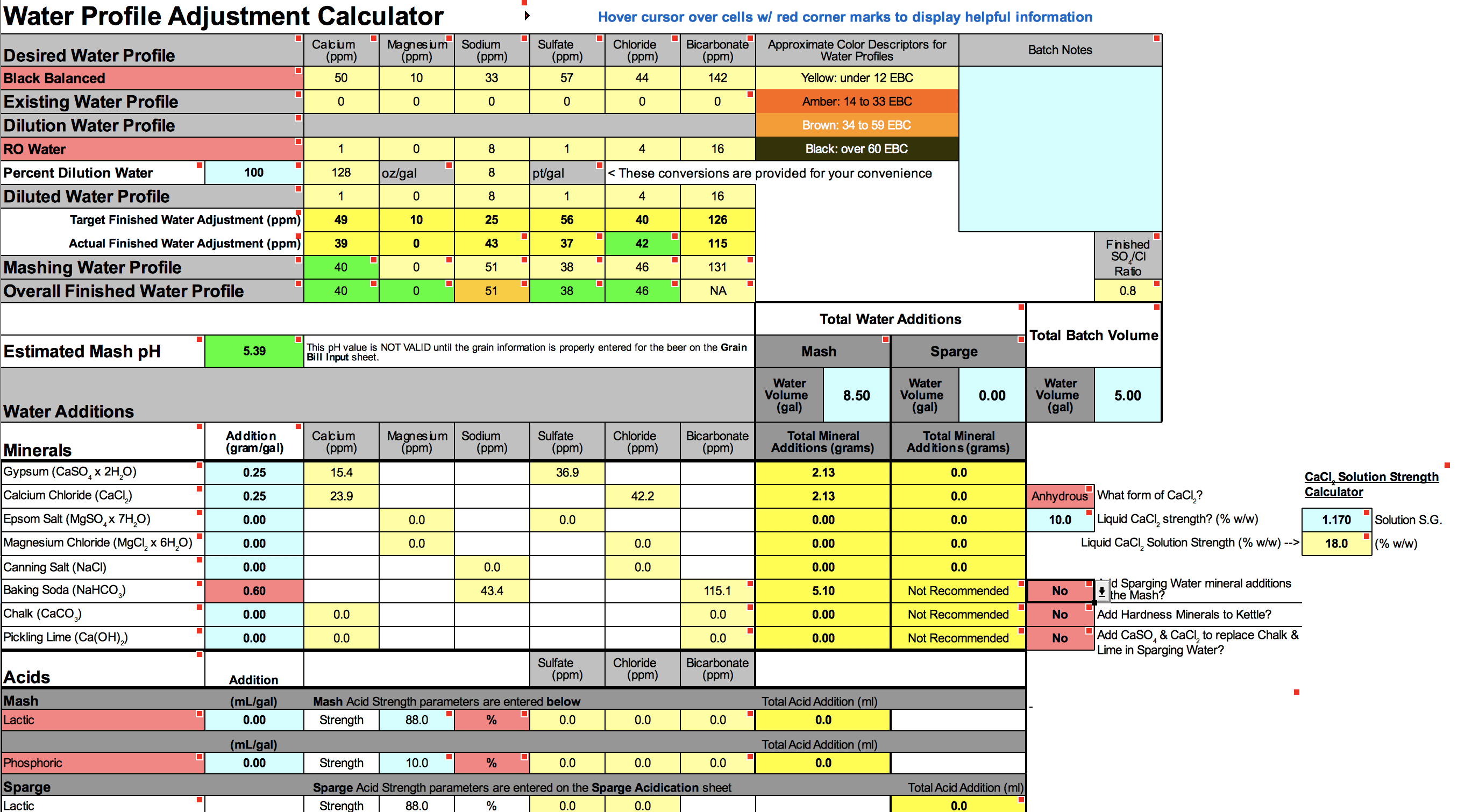
"... the cell will turn RED if too much of a mineral is added and the actual water adjustment of an ion differs from the target water adjustment of an ion by more than 10 ppm."
I learned long ago that one should look at ion concentration errors geometrically rather than arithmetically because in nature things (like your taste buds, eyes and ears) respond to the logarithm of the stimulus, not its absolute level. Hence audiophiles reckon in dB and photographers in stops. Furthermore what happens chemically is best described by the chemical potential of an ion and that is calculated from the log of its concentration.
Your target sodium level for amber bitter is 10 ppm. By adding the baking soda, your adjusted water will have 25 ppm of sodium. That is more than 10 ppm difference, hence the NaHCO3 cell turns red.
Is 10 ppm a significant error? If your target is 300 ppm sulfate, for example, and you get 310, certainly not. The log error is log(310/300) = log(310) - log(300) = 0.0142 and is certainly not a significant error (1%). OTOH if your goal is 10 mg/L and you get 25 you have more than doubled the sodium and the log error is log(25) - log(10) = 0.398. Engineers like to multiply log errors by 10 giving a unit called the decibel (after Alexander Graham Bell) so this is 3.98 dB. Three dB represents a significant change i.e. one which is almost always detectable. It is the size of a photographers stop, for example and represents a doubling. Smaller errors/changes are detectable but there is generally no question about the fact that 3 db represents a significant change. The 0.142 dB change associated with 310 mg/L vs 300 mg/L is not significant.
I suppose you can ignore the red cell, as I believe 25 ppm of sodium will be barely noticeable. It's up to you though how you want to proceed.
Tracking errors geometrically is great above some threshold. As noted here going from 10 to 25 might not that glaring a change in a taste test. Where it really shows its strength is in more sophisticated programs that look at finding the set of salt additions which best matches a desired target ion profile. Clearly a geometric error criterion is called for here as we wouldn't want sodium at the mg level to dominate the additions where some target levels are at appreciably higher levels. For example in synthesizing the water in the original post it is possible to get a solution in which the peak error in any ion concentration is 9.3% if minimizing the rms log errors whereas if one minimizes the rms ppm the peak error (in sodium) is 33.6% and on Mg is 21.4%.
I couldn't find where one enters the desired pH for the target water (nor for the source water either) so those must be on another page because one cannot do a synthesis without knowing the target pH. I assumed 7 in the calculations above. Also, if one is going to have carbonate alkalinity he must add bicarbonate or carbonate and those will raise the pH of the water. To get to target pH, unless it is high, acid must be added and in the calculations above I used phosphoric as I assumed hydrochloric is not available to most home brewers. If you allow me the use of hydrochloric I can hit the profile exactly (< 0.1% error in every ion) by adding to each liter of water
CaCl2.0H2O 39.69 mg/L
NaCl 0
CaSO4.2H20 90.85
MgSO4.7H2O 151.12
CaCO3 51.20
NaHCO3 36.54
Ca(OH)2 0
HCl 25.34
Add all the salts to the water. The CaCO3 won't all dissolve. Add the acid with stirring. The CaCO3 will dissolve and the pH will lower to 7.
Alternatively you can add
CaCl2.0H2O 71.21 mg/L
NaCl 7.43
CaSO4.2H20 90.89
MgSO4.7H2O 152.11
CaCO3 0
NaHCO3 25.86
Ca(OH)2 16.87
The pH will be over 10. Now you bubble CO2 through the water until the pH reaches 7. This is equivalent to adding 28.11 mg of CO2 to each liter of water.
Any time you want to synthesize a profile with alkalinity you have to add bicarbonate to the water which will bring the pH to close to 8.3. You can add the bicarbonate as carbonate plus acid. In either case you will need acid (more acid if carbonate was used) to lower the pH into any reasonable range. If emulating a natural water keep in mind that nature's acid is CO2.