- Joined
- Feb 20, 2011
- Messages
- 395
- Reaction score
- 92
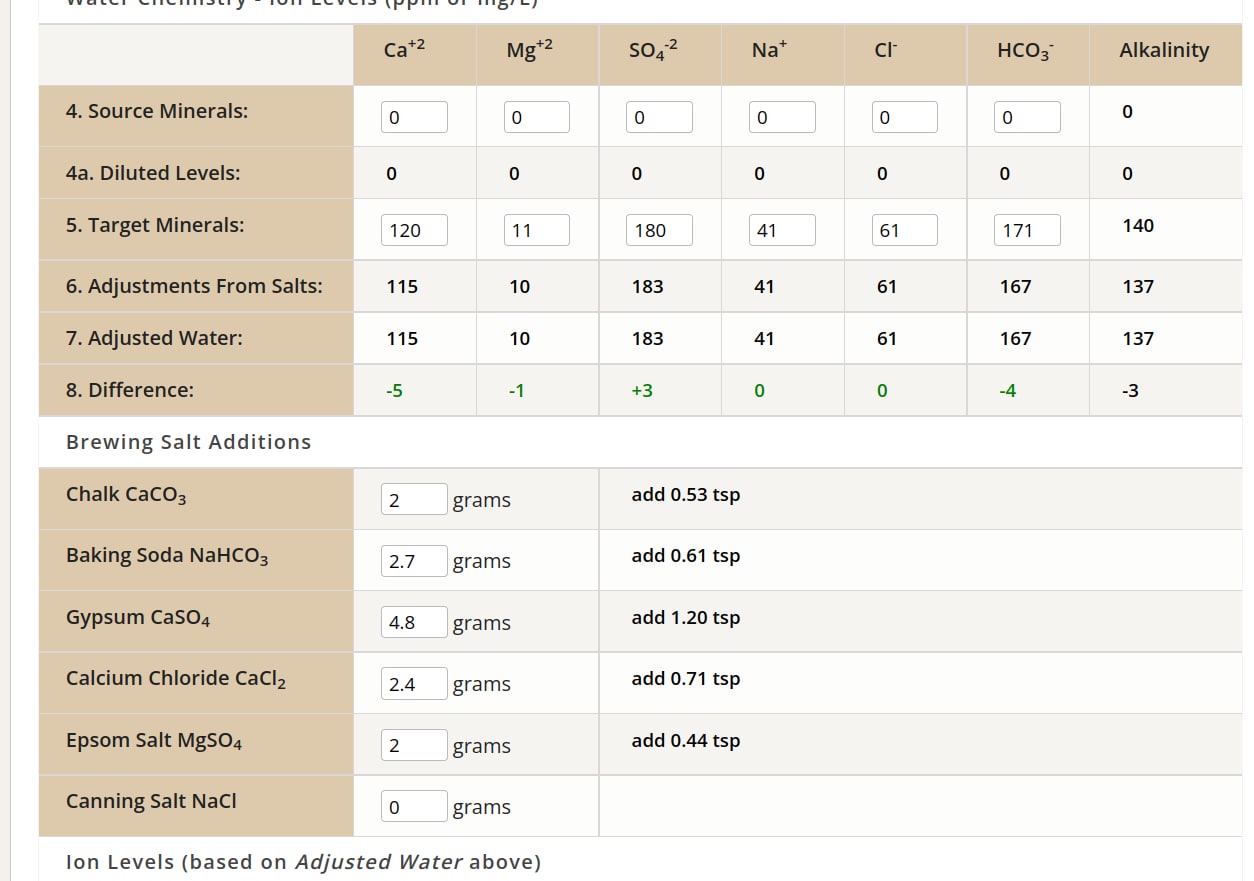
Hello! I’ve been promising SWMBO to put 5 gallon batches of homemade water on tap from RO. Any 5 gallon water chemistry recipes to build up from RO using our regular brewing salts?
Thanks!
Thanks!