william_shakes_beer
Well-Known Member
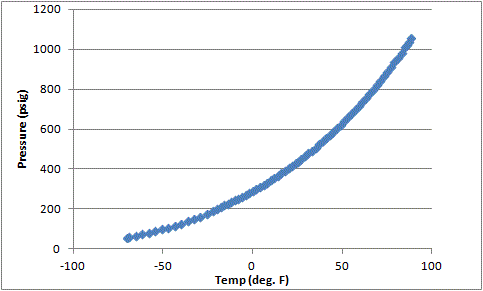
Bolted together my Keezer this weekend. Came down stairs to check it this AM. Opened the lid, and was shocked to find the brand new co2 bottle had dropped from 800 PSI to 600 PSI!! Oh crap, at this rate the 10# bottle will only last 4 days NO WAY!!! After stressing for about an hour I rememberated back to high school chemistry and the gas law. Given a volume of co2, pressure will fall as temperature is reduced. I turned on the freezer and dropped the temp from 68F to 36F overnight. 
Does anyone know the volume of co2 in a 10# tank at 800 PSI at 68F? I'd like to poke my temp drop in and verify the current reading does not suggest a leak. I found this calculator: http://www.1728.org/combined.htm I assume it needs the gas volume at atmospheric pressure. I have no idea how to calculate that. I have a 10# cylinder that at 68F holds 800#

Does anyone know the volume of co2 in a 10# tank at 800 PSI at 68F? I'd like to poke my temp drop in and verify the current reading does not suggest a leak. I found this calculator: http://www.1728.org/combined.htm I assume it needs the gas volume at atmospheric pressure. I have no idea how to calculate that. I have a 10# cylinder that at 68F holds 800#