You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Yet more evidence that commercial brewers do not mash at 5.2 to 5.6 pH ...
- Thread starter Silver_Is_Money
- Start date

Help Support Homebrew Talk:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
Robert65
Major Obvious (recently promoted)
No, it's local here. But an oddity. They appear all over one, and only one, county near me, and I get a real kick out them. Show me just one transparent hill! [emoji23]@Robert65, is that sign in your profile from Hawaii? I saw it while on vacation in January.
Like many things, gelatinization occurs over a wide temperature range, it’s just slower at lower temps, and you can get complete gelatinization below the “Gelatinization Temperature” given enough time. The Gelatinization Temperature is determined by a specific test, where the grain is heated up at a relatively high ramp rate, and the heat of reaction of the gelatinization is measured (using a method known as “Differential Scanning Calorimetry”.) The magnitude of the instantaneous DSC output is proportional to the instantaneous gelatinization rate. It starts out low at low temps and increases as the temp increases. The output reaches a peak at some temperature, and then starts going back down as the temp increases further. The drop off is because the gelatinization rate starts going down when most of the starch has been gelatinized. The temp at the peak of the DSC curve is defined as the gelatinization temperature. If you ran the test at a slower temp ramp rate, the gelatinization temp would come out lower, and at a higher ramp rate the gel temp would come out higher.And yet we can measure conversion, and see that a significant portion of it is achieved below gelatinization temperature.... Looking at the record of just my last mash, which I have on hand, dough in at 135°F, ramp 2°F/min to 144° (gelatinization temperature of this particular lot of malt is 147°F,) after a 20 minute rest I was at 73% maximum first wort density, 30 minutes at 147°F got to 94%, 30 minutes at 162 to 100%.
I'll look through some articles when I get a chance. I know I have some actual information on this question of just how much starch is available when.
Brew on

This is not a very good analogy. Corn pops instantaneously because of a steam explosion inside the kernel. Gelatinization occurs gradually starting at the surface of a starch granule, and progresses inwards towards the center of the kernel. Gelatinized starch chains are basically surrounded by water (a chain can be partially gelatinized.)Picture gelatinization just like popcorn before and after being popped. You start with very hard steely kernels, which would be very difficult to eat -- might break your teeth, etc. But when gelatinized, the kernels explode into the much larger fluffy stuff we know as popcorn. You could try to eat the hard kernels without gelatinization and without chipping a tooth if you were to grind the hard kernels into flour. The effect in your stomach then were you to eat this flour would be about the same as eating popped popcorn -- instead of fluffy popcorn melting down into starch soup in your gullet, you’d right away have corn flour soup, which accomplishes the same thing, and your body would really see no difference between the two. But, if you instead only broke the kernels into 6 or 7 pieces each, like a brewer’s mill does, but didn’t grind it down to powdery flour, then not only would it be much more difficult to eat than the flour or the popcorn, but your body would find it more difficult to digest.
So this is all sort of an analogy for the difference between ungelatinized starch vs. gelatinized. Gelatinization is explosively beneficial to the enzymes, increasing surface area by thousands of times. Certainly, broken kernels are better than unbroken ones… but not by much. Flour would be just about as beneficial as gelatinization, but we don’t get a ton of flour out of a brewer’s mill typically. And, it could be argued that even flour isn’t quite as beneficial as gelatinization. Gelatinization is also probably the most energy efficient way to make the most starches available to the enzymes.
So…… that’s my story and I’m sticking to it, for now.
Enzymes are large, complex, folded protein molecules that have a single active site somewhere on the outer portion of the molecular envelop. In order for the enzyme to do it’s job, this active site has to align itself with the bond in the starch chain that it is going to break (hydrolyze.). This can only happen if the enzyme is floating around in water, and is free to move around and reorient itself. The enzymes cannot penetrate into the ungelatinized part of a starch grain. Also in order to hydrolyze (break) a starch bond, a molecule of water is required to be present in order to react with the bond being broken. No water at the bond site, no hydrolysis.
Brew on

Like many things, gelatinization occurs over a wide temperature range, it’s just slower at lower temps, and you can get complete gelatinization below the “Gelatinization Temperature” given enough time. The Gelatinization Temperature is determined by a specific test, where the grain is heated up at a relatively high ramp rate, and the heat of reaction of the gelatinization is measured (using a method known as “Differential Scanning Calorimetry”.)
Brew on
So the rate of gelatinization is function of both time and temperature. I envisioned it being an all or none phenomenon that occurred at the gelatinization temp. Thanks, this helps my understanding greatly. It now makes sense to me that having a rest slightly below the gelatinization temp is not a total waste of beta amylase.
Gnomebrewer
Well-Known Member
A little off topic here, but I recently found out that whirlfloc tabs have adipic acid, Sodium carbonate and Sodium bicarbonate in them (at least, the brand I use does). Has anyone looked at how they affect pH?

$176.97
1pc Commercial Keg Manifold 2" Tri Clamp,Ball Lock Tapping Head,Pressure Gauge/Adjustable PRV for Kegging,Fermentation Control
hanhanbaihuoxiaoshoudian

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Gas MFL)
Guangshui Weilu You Trading Co., Ltd

$33.95
Five Star - 6022b_ - Star San - 32 Ounce - High Foaming Sanitizer
Bridgeview Beer and Wine Supply

$7.79 ($7.79 / Count)
Craft A Brew - LalBrew Voss™ - Kveik Ale Yeast - For Craft Lagers - Ingredients for Home Brewing - Beer Making Supplies - (1 Pack)
Craft a Brew

$22.00 ($623.23 / Ounce)
AMZLMPKNTW Ball Lock Sample Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging joyful
无为中南商贸有限公司

$10.99 ($31.16 / Ounce)
Hornindal Kveik Yeast for Homebrewing - Mead, Cider, Wine, Beer - 10g Packet - Saccharomyces Cerevisiae - Sold by Shadowhive.com
Shadowhive
![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)
$6.95 ($17.38 / Ounce)
$7.47 ($18.68 / Ounce)
Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]
Hobby Homebrew

$58.16
HUIZHUGS Brewing Equipment Keg Ball Lock Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging Brewing Equipment
xiangshuizhenzhanglingfengshop

$33.99 ($17.00 / Count)
$41.99 ($21.00 / Count)
2 Pack 1 Gallon Large Fermentation Jars with 3 Airlocks and 2 SCREW Lids(100% Airtight Heavy Duty Lid w Silicone) - Wide Mouth Glass Jars w Scale Mark - Pickle Jars for Sauerkraut, Sourdough Starter
Qianfenie Direct

$172.35
2 Inch Tri Clamp Keg Manifold With Ball Lock Posts, Pressure Gauge, PRV (0-30 PSI) – Homebrew, Fermentation, Kegging System
wuhanshijiayangzhiyimaoyiyouxiangongsi

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid Hose Barb)
yunchengshiyanhuqucuichendianzishangwuyouxiangongsi
lowtones84
Well-Known Member
A little off topic here, but I recently found out that whirlfloc tabs have adipic acid, Sodium carbonate and Sodium bicarbonate in them (at least, the brand I use does). Has anyone looked at how they affect pH?
I've been kind of wondering the same. I haven't been able to cool samples fast enough from boiling to really adjust in time (admittedly haven't tried all that hard) but whirlfloc -always- makes a noticeable difference for me. I've no doubt it may work better at about 5.0 but it sure seems to be effective regardless.
Thanks all for the great discussion in this thread. That mash made easy calculator may be the missing piece I need to do kettle ph adjustment. For now I'll still be targeting around 5.4 mash ph with my system, but will try the kettle adjustment on the next few batches.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
A little off topic here, but I recently found out that whirlfloc tabs have adipic acid, Sodium carbonate and Sodium bicarbonate in them (at least, the brand I use does). Has anyone looked at how they affect pH?
I've never weighed one, but a Whirlfloc tablet is likely (as a guess) to weight about 6 grams. If the bulk of that is Irish Moss as purified Kappa Carrageenan, the chemicals of your concern are likely present at very low levels.
Last edited:
Die_Beerery
Well-Known Member
- Joined
- Aug 21, 2017
- Messages
- 842
- Reaction score
- 643
Correct, they don't.I've never weighed one, but a Whirlfloc tablet is likely (as a guess) to weight about 6 grams. If the bulk of that is Irish Moss as purified Kappa Carrageenan, the chemicals of your concern are likely present at very low levels.
Dustin_J
Well-Known Member
Of late I've been combing through peer reviewed dissertations on "Limit Dextrinase", which is an enzyme that can convert starches which are not convertible via alpha or beta amylase into fermentable sugars. It seems that the room temperature measured mash pH range which most promotes the action of Limit Dextrinase is 5.0 to 5.5, with a slight peak at 5.5. Thus if maximum conversion is desired via maximizing Limit Dextrinase, a somewhat lower mash pH target may prove to be the ticket, but I would intuitively presume that this may lead to thinner beer from the perspective of mouthfeel.
If you haven't ran across this, you might find it interesting: https://www.acervapetropolis.com.br/download/Barley_and_malt_Starch_in_brewing__a_review_0515-01.pdf
Bamforth also discusses a similar slightly lower pH target for maximizing the impact of Limit Dextrinase and, potentially, fermentability, but he also notes that Limit Dextrinase is generally not very "free" or accessible in wort (barring fairly extreme measures such as hammer milling). Interestingly, he also comments that it's likely more temp stable than Beta.
Last edited:
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
A good read. The article states that much of limit dextrinase is "bound" and "inhibited" within malt, and the trick of achieving greater levels of fermentation is to discover ways to unbind it as well as to free it from being inhibited. Pulverizing and pH lowering are two known methods to achieve the goal.
twd000
Well-Known Member
I came across this in an article about German Hefeweizen beers:
"High salt contents can reduce the desired soft taste of the beer. Therefore, it is advisable to desalinate the water rather than using brewing salts to increase the mineral content. Considering the aforementioned reasons, a mash or wort acidification is normally not carried out. It has been observed that an acidified mash results in lower quality estery aromas [Her05]. Wort acidification 10 minutes before the end of boil can increase the phenolic and fresh character of the beer, according to Drexler (Weißbierbrauerei Schneider-Weisse)."
http://braumagazin.de/article/brewing-bavarian-weissbier-all-you-ever-wanted-to-know/
has anyone tried this? Notice any difference in esters, phenolics, or "fresh character of the beer"?
At the homebrew scale, is it just as simple as skipping mash pH adjustment and doing the only adjustment 10 minutes before the end of the boil?
"High salt contents can reduce the desired soft taste of the beer. Therefore, it is advisable to desalinate the water rather than using brewing salts to increase the mineral content. Considering the aforementioned reasons, a mash or wort acidification is normally not carried out. It has been observed that an acidified mash results in lower quality estery aromas [Her05]. Wort acidification 10 minutes before the end of boil can increase the phenolic and fresh character of the beer, according to Drexler (Weißbierbrauerei Schneider-Weisse)."
http://braumagazin.de/article/brewing-bavarian-weissbier-all-you-ever-wanted-to-know/
has anyone tried this? Notice any difference in esters, phenolics, or "fresh character of the beer"?
At the homebrew scale, is it just as simple as skipping mash pH adjustment and doing the only adjustment 10 minutes before the end of the boil?
Bilsch
Well-Known Member
- Joined
- May 4, 2015
- Messages
- 1,754
- Reaction score
- 1,609
I came across this in an article about German Hefeweizen beers:
"High salt contents can reduce the desired soft taste of the beer. Therefore, it is advisable to desalinate the water rather than using brewing salts to increase the mineral content. Considering the aforementioned reasons, a mash or wort acidification is normally not carried out. It has been observed that an acidified mash results in lower quality estery aromas [Her05]. Wort acidification 10 minutes before the end of boil can increase the phenolic and fresh character of the beer, according to Drexler (Weißbierbrauerei Schneider-Weisse)."
http://braumagazin.de/article/brewing-bavarian-weissbier-all-you-ever-wanted-to-know/
has anyone tried this? Notice any difference in esters, phenolics, or "fresh character of the beer"?
At the homebrew scale, is it just as simple as skipping mash pH adjustment and doing the only adjustment 10 minutes before the end of the boil?
Yes the Beerery has written about this very topic.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
In the below linked peer reviewed document it will be seen in "Table IV" on page 136 that "Mash pH" (for all but the two grist "controls" on either end) was adjusted to pH 5.4 as measured at a specified temperature of 50 degrees C. (122 degrees F.), per acid adition quantities computed via the use of the Kunze method. Note that the consequence of this was that later on downstream the "Wort pH" (as measured at a specified 20 degrees C., or 68 degrees F.) was for all cases in the range of 5.8-5.9 pH, so as to totally verify that had the mash pH also been measured at 20 degrees C. it would have had to have been well above 5.4. This is yet another proof that these professionals are mashing at well above the pH range thought to be optimal by amateur home brewers. And if mash acidification here was done in compliance with the Kunze method, then he likewise would be presumed to have standardized on measuring mash pH at 50 degrees C.
https://www.onlinelibrary.wiley.com/doi/epdf/10.1002/j.2050-0416.2004.tb00192.x
https://www.onlinelibrary.wiley.com/doi/epdf/10.1002/j.2050-0416.2004.tb00192.x
Die_Beerery
Well-Known Member
- Joined
- Aug 21, 2017
- Messages
- 842
- Reaction score
- 643
In the below linked peer reviewed document it will be seen in "Table IV" on page 136 that "Mash pH" (for all but the two grist "controls" on either end) was adjusted to pH 5.4 as measured at a specified temperature of 50 degrees C. (122 degrees F.), per acid adition quantities computed via the use of the Kunze method. Note that the consequence of this was that later on downstream the "Wort pH" (as measured at a specified 20 degrees C., or 68 degrees F.) was for all cases in the range of 5.8-5.9 pH, so as to totally verify that had the mash pH also been measured at 20 degrees C. it would have had to have been well above 5.4. This is yet another proof that these professionals are mashing at well above the pH range thought to be optimal by amateur home brewers. And if mash acidification here was done in compliance with the Kunze method, then he likewise would be presumed to have standardized on measuring mash pH at 50 degrees C.
https://www.onlinelibrary.wiley.com/doi/epdf/10.1002/j.2050-0416.2004.tb00192.x
What are we trying to get at?
If its about a higher mash pH, then do it. Mash one beer at "normal" and one higher. Having mashed beers at all pH's I have my conclusion. But, perhaps, yours may vary. Higher mash pH beers for me.. no bueno.
There are few things that irk me more than theoretical discussion! More do, less talk!
Last edited:
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
What are we trying to get at?
If its about a higher mash pH, then do it. Mash one beer at "normal" and one higher. Having mashed beers at all pH's I have my conclusion. But, perhaps, yours may vary. Higher mash pH beers for me.. no bueno.
There are few things that irk me more than theoretical discussion! More do, less talk!
Do you subsequently adjust at some juncture during the boil to ~5.1 pH (for room temperature wort)?
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
There are few things that irk me more than theoretical discussion! More do, less talk!
I'm approaching being old enough to be your grandfather. Things tend to move a lot more slowly at my age. I've moved to more of the theoretical and research side of things.
Die_Beerery
Well-Known Member
- Joined
- Aug 21, 2017
- Messages
- 842
- Reaction score
- 643
Do you subsequently adjust at some juncture during the boil to ~5.1 pH (for room temperature wort)?
Only at knock out. *Mostly* mash and boil at 5.4 room temp (PH at process temp, is less and varies).
Die_Beerery
Well-Known Member
- Joined
- Aug 21, 2017
- Messages
- 842
- Reaction score
- 643
Nah, that means you now have MORE time to do!I'm approaching being old enough to be your grandfather. Things tend to move a lot more slowly at my age. I've moved to more of the theoretical and research side of things.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
NOTE: I'm now more highly inclined to believe that for brewers of such magnitude as the likes of Kunze and Narziss the nominal standard mash pH of 5.40 was (for them) intended to be measured at 50 degrees C., (122 degrees F.) as it seems that an initial rest at ~122 degrees F. was quite typically undertaken for say 10-20 minutes back in the day when these giants laid down the mash pH rules. This somewhat noticeably changes my earlier opinion as represented within this thread that in the heyday of these brewing giants Mash pH was taken at full single infusion mash temperatures within the range of 148 to 158 degrees F. And as the upward pH shift (inherent to your meter, with due allowance for meter variance as to temperature changes here) witnessed in going from 122 degrees F. to 68 degrees F. as the baseline for taking mash pH readings will not be as dramatic as would be the upward pH shift for going from ballpark 153 degrees F. to 68 degrees F., I now see targeting a room temperature measured mash pH of 5.55 as being more the ideal, whereas prior to now (as seen in my opinion expressed throughout this thread) I had been leaning toward as high as 5.65 pH as the ideal. It also means that the pH reading must be taken within the window of the duration of a typical 122 degree F. rest, and I believe that this is where sampling at 10-20 minutes into the mash as the norm for grabbing a mash pH reading also seems to find its origin.
Last edited:
Die_Beerery
Well-Known Member
- Joined
- Aug 21, 2017
- Messages
- 842
- Reaction score
- 643
NOTE: I'm now more highly inclined to believe that for brewers of such magnitude as the likes of Kunze and Narziss the nominal standard mash pH of 5.40 was (for them) intended to be measured at 50 degrees C., (122 degrees F.) as it seems that an initial rest at ~122 degrees F. was quite typically undertaken for say 10-20 minutes back in the day when these giants laid down the mash pH rules. This somewhat noticeably changes my earlier opinion as represented within this thread that in the heyday of these brewing giants Mash pH was taken at full single infusion mash temperatures within the range of 148 to 158 degrees F. And as the upward pH shift (inherent to your meter, with due allowance for meter variance as to temperature changes here) witnessed in going from 122 degrees F. to 68 degrees F. as the baseline for taking mash pH readings will not be as dramatic as would be the upward pH shift for going from ballpark 153 degrees F. to 68 degrees F., I now see targeting a room temperature measured mash pH of 5.55 as being more the ideal, whereas prior to now (as seen in my opinion expressed throughout this thread) I had been leaning toward as high as 5.65 pH as the ideal. It also means that the pH reading must be taken within the window of the duration of a typical 122 degree F. rest, and I believe that this is where sampling at 10-20 minutes into the mash as the norm for grabbing a mash pH reading also seems to find its origin.
I do not concur.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
I captured this chart from one of Kai Troester's (Braukaiser's) many web pages (linked below the chart), and he got it from a 2004 book by Briggs:
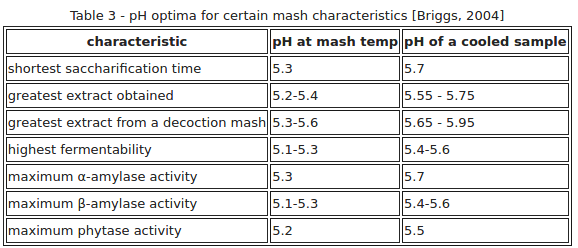
http://www.braukaiser.com/wiki/index.php/Starch_Conversion
Briggs presumed that the differential between mash temperature measured and room temperature measured pH's for Wort is 0.35 points. I believe Palmer presumed 0.25 points. And some say they observe more on the order of 0.15 points. All said though, this appears to appreciably strengthen the case for the room temperature measured optimal midrange mash pH target clearly being higher than the home brewing worlds most often presumed nominal midrange ideal of 5.4.

http://www.braukaiser.com/wiki/index.php/Starch_Conversion
Briggs presumed that the differential between mash temperature measured and room temperature measured pH's for Wort is 0.35 points. I believe Palmer presumed 0.25 points. And some say they observe more on the order of 0.15 points. All said though, this appears to appreciably strengthen the case for the room temperature measured optimal midrange mash pH target clearly being higher than the home brewing worlds most often presumed nominal midrange ideal of 5.4.
Last edited:
Die_Beerery
Well-Known Member
- Joined
- Aug 21, 2017
- Messages
- 842
- Reaction score
- 643
I captured this chart from one of Kai Troester's (Braukaiser's) many web pages (linked below the chart), and he got it from a 2004 book by Briggs:
View attachment 670544
http://www.braukaiser.com/wiki/index.php/Starch_Conversion
Briggs presumed that the differential between mash temperature measured and room temperature measured pH's for Wort is 0.35 points. I believe Palmer presumed 0.25 points. And some say they observe more on the order of 0.15 points. All said though, this appears to appreciably strengthen the case for the room temperature measured optimal midrange mash pH target clearly being higher than the home brewing worlds most often presumed nominal midrange ideal of 5.4.
Many have actually done the comparison. Taylor measured the differential to be 0.25.
But admittedly not over and over repeatedly. Nevertheless, 0.25 is a good ballpark that falls right in the middle of all the actual reports anyway so I'm sticking with it for now. I don't care about hundredths of a unit anyway. Even a tenth... may be significant.
But admittedly not over and over repeatedly. Nevertheless, 0.25 is a good ballpark that falls right in the middle of all the actual reports anyway so I'm sticking with it for now. I don't care about hundredths of a unit anyway. Even a tenth... may be significant.
Die_Beerery
Well-Known Member
- Joined
- Aug 21, 2017
- Messages
- 842
- Reaction score
- 643
Taylor as in the Royal Taylor, you?
At what temp? My offsets change based on mash temps. So, since I step mash (normally 5 steps) my offsets change 5 times. Oh, and they vary on grist and other environmental factors. So....
At what temp? My offsets change based on mash temps. So, since I step mash (normally 5 steps) my offsets change 5 times. Oh, and they vary on grist and other environmental factors. So....
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
A.J. deLange, 1998The DeClerk publishes a shift of about 0.3 pH units. In my own brewing I
see a shift of about half that. I solicited measurement data from other
brewers and got data from only one respondent who actually measured a
mash (and got similar results) and from another who did measurements
with malt samples on the bench (and got about 0.3).
http://hbd.org/hbd/archive/2788.html
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Temperature is inherent within the Nernst Equation, and this assures that pH will shift upward as temperature falls.
Die_Beerery
Well-Known Member
- Joined
- Aug 21, 2017
- Messages
- 842
- Reaction score
- 643
Ok, so I'll get to my point.
My point is.... Tracking mash temp pH is stupid. Everything is conflicting, because inherently it itself is conflicting.
Measure at room temp, and adjust at room temp. Because no one has ANY idea of what mash temp ph's actually are in any given scenario. It varies brewer to brewer, mash to mash, and temp, etc.. It doesn't matter, oh and the term "probrewers" these days holds about as much weight as the ATF stamp they need to pay to be one.
My point is.... Tracking mash temp pH is stupid. Everything is conflicting, because inherently it itself is conflicting.
Measure at room temp, and adjust at room temp. Because no one has ANY idea of what mash temp ph's actually are in any given scenario. It varies brewer to brewer, mash to mash, and temp, etc.. It doesn't matter, oh and the term "probrewers" these days holds about as much weight as the ATF stamp they need to pay to be one.
Taylor as in the Royal Taylor, you?
At what temp? My offsets change based on mash temps. So, since I step mash (normally 5 steps) my offsets change 5 times. Oh, and they vary on grist and other environmental factors. So....
I'll never claim to be Royal, but yes. Admittedly, more experiments are needed. And *I* am not worried about dunking my pH meter into a hot mash, doesn't bother me one bit.
IF it matters so much how I do things... I'm a single infusion masher, similar to about 90% of all homebrewers, almost always at a reasonable 148-152 F (about 65-66 C) for at least 40 minutes. I measure after about the first 10 minutes of the mash, and if adjustment is needed, add acid or pickling lime, mix well, and measure again 5-10 minutes later.
Or in many other cases, I don't even bother to measure pH at all, because, like, I dunno how much it really matters anyway.
Die_Beerery
Well-Known Member
- Joined
- Aug 21, 2017
- Messages
- 842
- Reaction score
- 643
If I am not mistaken, you use cheap like $10 amazon meters right? One issue is probe life sure, another issue is if your meter can even read pH at that temp. Many pH probes can't read above like 120f.
So. Theres that as well..
But as you said, not much matters anyways. Except, of course, for people who it does.
Context.
So. Theres that as well..
But as you said, not much matters anyways. Except, of course, for people who it does.
Context.
Similar threads
- Replies
- 7
- Views
- 1K
- Replies
- 6
- Views
- 1K
- Replies
- 6
- Views
- 1K
- Replies
- 17
- Views
- 5K














































