@TheMadKing I wasn't as clear in my reply as I intended to be. I can see that you have a good understanding of what I was trying to convey. Thank you for pointing this out.Sorry, I'm not trying to be dense, but your original statement did not contain a sample taken at 77F/25C - it contained a value calculated at that temperature, and then a sample taken and measured at a different temperature, which is all I'm confused about
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Yet more evidence that commercial brewers do not mash at 5.2 to 5.6 pH ...
- Thread starter Silver_Is_Money
- Start date

Help Support Homebrew Talk:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
- Joined
- Apr 13, 2013
- Messages
- 1,987
- Reaction score
- 965
@TheMadKing you miss the point and so the confusion.
The pH meter is first calibrated with calibration solutions at 77F/25C for the sample taken at 77F/25C .
It is again calibrated with calibration solutions at 152F/66.7C for the sample taken at 152F/66.7C.
I hope you are manually calibrating your pH meter at the higher temperatures to the pH value of the buffer solution at that temperature. While some buffers are relatively stable, there are others that swing a couple of tenths pH between 20C and 50C.
Wow! I cannot believe that was not clear in my original post and later reply. That is exactly what I meant @Oginme.I hope you are manually calibrating your pH meter at the higher temperatures to the pH value of the buffer solution at that temperature.
Why so much confusion remains around such a straight forward concept is beyond me. I hope the following is clearer and easier to understand now.
“The pH meter is first calibrated with calibration solutions at 77F/25C for a sample taken at the same 77F/25C temperature.
The pH meter is then calibrated with calibration solutions at 152F/66.7C for a sample taken at the higher 152F/66.7C temperature.”
Last edited:
Wow! I cannot believe that was not clear in my original post and later reply. That is exactly what I meant @Oginme.
Why so much confusion remains around such a straight forward concept is beyond me.
FWIW I thought that part was explicitly stated
I’m really interested in this mashing at higher pH and then acidifying in kettle concept. I believe most of the recommendations I’ve been previously following aim to adjust mash pH to about 5.3 - 5.4 in order to make sure kettle pH ends up in the 5.0-5.2 range. Current batch I mashed kinda high and added acid at end of boil to bring pH down to 5.2. Next batch I’ll keep grist same and add that extra acid at beginning of boil.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
A week or so ago I bumped all of my recipes up to mash at a room temperature measured 5.6 pH. Today I bumped all of them up to mash at a room temperature measured 5.65 pH. That should plant me within a mash temperature measured pH window of ~5.3 to ~5.4. Then downstream of the mash and lautering/run-off (and pre-boil) I will measure the pH at room temperature (20 Deg. C.) and acid adjust (also pre-boil) so as to hit a post boil and cooling target of 5.10 pH.
Last edited:

$2.41
$29.95
Mastering Homebrew: The Complete Guide to Brewing Delicious Beer (Beer Brewing Bible, Homebrewing Book)
Clickgoodwill

$7.79 ($7.79 / Count)
Craft A Brew - LalBrew Voss™ - Kveik Ale Yeast - For Craft Lagers - Ingredients for Home Brewing - Beer Making Supplies - (1 Pack)
Craft a Brew
![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)
$6.95 ($17.38 / Ounce)
$7.47 ($18.68 / Ounce)
Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]
Hobby Homebrew

$39.22 ($39.22 / Count)
Brewer's Best Home Brew Beer Ingredient Kit - 5 Gallon (Mexican Cerveza)
Amazon.com

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Gas MFL)
Guangshui Weilu You Trading Co., Ltd

$176.97
1pc Commercial Keg Manifold 2" Tri Clamp,Ball Lock Tapping Head,Pressure Gauge/Adjustable PRV for Kegging,Fermentation Control
hanhanbaihuoxiaoshoudian

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid Hose Barb)
yunchengshiyanhuqucuichendianzishangwuyouxiangongsi

$22.00 ($623.23 / Ounce)
AMZLMPKNTW Ball Lock Sample Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging joyful
无为中南商贸有限公司

$479.00
$559.00
EdgeStar KC1000SS Craft Brew Kegerator for 1/6 Barrel and Cornelius Kegs
Amazon.com

$58.16
HUIZHUGS Brewing Equipment Keg Ball Lock Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging Brewing Equipment
xiangshuizhenzhanglingfengshop

$10.99 ($31.16 / Ounce)
Hornindal Kveik Yeast for Homebrewing - Mead, Cider, Wine, Beer - 10g Packet - Saccharomyces Cerevisiae - Sold by Shadowhive.com
Shadowhive
Robert65
Major Obvious (recently promoted)
Best practice is not to acidify right at the beginning of the boil. You want to conduct at least part of the boil at a higher pH to optimize DMS formation from SMM, and hop utilization. It is necessary to acidify to a level sufficient to give a post boil, chilled wort pH of 5.0-5.2 in order for carrageenan based kettle finings to function, and for proper fermentation performance. Kunze recommends acidification "shortly before the end of boiling."
Yah I’m thinking about Martin’s comment regarding beers mashed too high pH being common cause of bad to mediocre beer. How many of those mashed too high were acidified down in the kettle. I’m thinking none of them.
Best practice is not to acidify right at the beginning of the boil. You want to conduct at least part of the boil at a higher pH to optimize DMS formation from SMM, and hop utilization. It is necessary to acidify to a level sufficient to give a post boil, chilled wort pH of 5.0-5.2 in order for carrageenan based kettle finings to function, and for proper fermentation performance. Kunze recommends acidification "shortly before the end of boiling."
Hmm good to know. Reason I’ve seen for earlier is to prevent wort darkening but I work with quite light worts so not too worried about that.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Best practice is not to acidify right at the beginning of the boil. You want to conduct at least part of the boil at a higher pH to optimize DMS formation from SMM, and hop utilization. It is necessary to acidify to a level sufficient to give a post boil, chilled wort pH of 5.0-5.2 in order for carrageenan based kettle finings to function, and for proper fermentation performance. Kunze recommends acidification "shortly before the end of boiling."
What about Maillard run-away control? Wouldn't that necessitate adding the acid before the boil? Perhaps the best compromise is to target 5.2 pH and add the acid pre-boil, rather than targeting 5.0 or 5.1 pH. ???
I believe this acidification step is also intended to promote an excellent level of "hot break" during the boil. Yet another reason to consider adding it before the boil is too far along.
Last edited:
- Joined
- Apr 13, 2013
- Messages
- 1,987
- Reaction score
- 965
Wow! I cannot believe that was not clear in my original post and later reply. That is exactly what I meant @Oginme.
Why so much confusion remains around such a straight forward concept is beyond me. I hope the following is clearer and easier to understand now.
“The pH meter is first calibrated with calibration solutions at 77F/25C for a sample taken at the same 77F/25C temperature.
The pH meter is then calibrated with calibration solutions at 152F/66.7C for a sample taken at the higher 152F/66.7C temperature.”
I got that part, but the pH of a buffer solution at the higher temperature is not the same as it is when it is at standard conditions (20C)
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
I got that part, but the pH of a buffer solution at the higher temperature is not the same as it is when it is at standard conditions (20C)
I agree. And if the true (as in factual) downward pH shift due to the heat is of great enough magnitude, some pH meters may even fail to "auto recognize" a 4.01 pH buffer as a 4.01 pH buffer, and thus fail to lock in on it and calibrate. Or they may see a 7.01 buffer as a 6.86 buffer and lock in at 6.86 instead of 7.01. Something to ponder....
Last edited:
Robert65
Major Obvious (recently promoted)
Yes, earlier acidification will reduce darkening of the wort. But I'd assume if that's a concern, you're using Pilsner malt, and so DMS elimination would be a priority also. Hop bitterness is said to be cleaner at the lower pH, but reduced utilization necessitates more hops, hence more vegetative matter, negating this effect. Kunze does balance these and other factors and decisively favors acidification late in the boil. Reducing the length and vigor of the boil -- best practice for many other reasons -- will do more than acidification to limit darkening of the wort. This will in turn be facilitated by rapidly dealing with DMS at the higher pH. Also, some formation of reductones (melanoidins etc.) is desirable for stabilization of the beer and at higher pH should be achieved better in the short, low intensity boil.What about Maillard run-away control? Wouldn't that necessitate adding the acid before the boil? Perhaps the best compromise is to target 5.2 pH and add the acid pre-boil, rather than targeting 5.0 or 5.1 pH. ???
I believe this acidification step is also intended to promote an excellent level of "hot break" during the boil. Yet another reason to consider adding it before the boil is too far along.
I can't find any information relating pH to break coagulation, but 5.0 is the target for kettle finings. Current practice, as also advocated by Kunze, does not favor as full a protein precipitation as was formerly recommended leading to the practice of longer more vigorous boiling. Carrying some high molecular weight proteins over into the cast wort greatly enhances foam qualities without compromising clarity of the beer.
hopjuice_71
Well-Known Member
- Joined
- Mar 26, 2016
- Messages
- 373
- Reaction score
- 374
Yah I’m thinking about Martin’s comment regarding beers mashed too high pH being common cause of bad to mediocre beer. How many of those mashed too high were acidified down in the kettle. I’m thinking none of them.
Really interesting that you should mention this. There was a thread not a whole lot different from this one a bit more than a year ago and similar comments came up. A big portion of my day job is enzymology (glycoside hydrolases like the amylases and proteases) and many of the thoughts regarding specific mash enzyme performance and pH didn't compute for me. I went and tracked down the research on the classes of enzymes in action in the mash and found that virtually all of them are highly active across a wide range of pHs, much more so than you see in the typical brewing tables.
This was met with some resistance. So I went further and started tracking down as much as I could that combined study of pHs in wort production and how it impacts the physicochemical and sensory properties of beer. I found nice studies where mashes were done at a single "optimal" pH, then the kettle pH was adjusted up or down. This was reported to have quite a profound influence on the resulting product. Then there were studies where mash pH was altered. This also had measurable outcomes. So I was quite convinced that pH is indeed a very important consideration. But in the latter set of studies, the kettle pH was not adjusted, so the results of the mash pH would track though the whole process (i.e. higher or lower kettle pHs as well). What I never found, and even asked people to point me to, are the studies that nail where the pH is may be most important - experiments where the mash pH is varied but the kettle pH is normalized to something considered optimal. If anyone here knows of these please let me know (@Robert65?). I'm kind of at the point where I'm postulating there is some leeway with mash pH that can be "corrected" by adjustment of the kettle pH.
Yes, earlier acidification will reduce darkening of the wort. But I'd assume if that's a concern, you're using Pilsner malt, and so DMS elimination would be a priority also. Hop bitterness is said to be cleaner at the lower pH, but reduced utilization necessitates more hops, hence more vegetative matter, negating this effect. Kunze does balance these and other factors and decisively favors acidification late in the boil. Reducing the length and vigor of the boil -- best practice for many other reasons -- will do more than acidification to limit darkening of the wort. This will in turn be facilitated by rapidly dealing with DMS at the higher pH. Also, some formation of reductones (melanoidins etc.) is desirable for stabilization of the beer and at higher pH should be achieved better in the short, low intensity boil.
I can't find any information relating pH to break coagulation, but 5.0 is the target for kettle finings. Current practice, as also advocated by Kunze, does not favor as full a protein precipitation as was formerly recommended leading to the practice of longer more vigorous boiling. Carrying some high molecular weight proteins over into the cast wort greatly enhances foam qualities without compromising clarity of the beer.
This post has brought things back into focus for me
What problem are you all trying to solve exactly by mashing at a higher pH?
Is it a purely academic exercise to match the intent of literature, or does it address specific issues in a tangible way?
I’ve noticed a slight tartness in some of my beers but not others. I’ve had dull malt flavors in some but not others. I’ve had muddled hop flavors in some but not others. And I’ve won a decent number of medals and generally consider my beer to be on the good end of the spectrum of homebrew with the odd obvious flaw mostly being clearly related to recipe issues, fermentation, or oxidation.
One if the very few things that ALL of these beers have had in common was a mash pH of 5.2-5.4 as calculated by bru’n water (so thats the room temp pH). So it would seem that it doesn’t really solve (or cause) any problems for me one way or the other.
Robert65
Major Obvious (recently promoted)
I'm kind of at the point where I'm postulating there is some leeway with mash pH that can be "corrected" by adjustment of the kettle pH.
Well I think that's it in a nutshell.
(I should say I've never heard of any kettle adjustment other than downward. If your mash pH is low enough to already provide a cast wort pH of ~5.0, no need. If your mash is any lower than that, it's a dumper probably anyway. Unless you're making something hazy and sour, in which case you don't care if the wort is below 5.0 and as a result it fails to fine.)
Robert65
Major Obvious (recently promoted)
This post has brought things back into focus for me
What problem are you all trying to solve exactly by mashing at a higher pH?
See the nutshell above. I think it is more accurate to say -- as @Silver_Is_Money has been for some time -- that we needn't worry so much about targeting a lower pH in the mash, we can just "let it ride." There really isn't a problem to solve there. What is most important is the post boil wort pH.
hopjuice_71
Well-Known Member
- Joined
- Mar 26, 2016
- Messages
- 373
- Reaction score
- 374
This post has brought things back into focus for me
What problem are you all trying to solve exactly by mashing at a higher pH?
Is it a purely academic exercise to match the intent of literature, or does it address specific issues in a tangible way?
I’ve noticed a slight tartness in some of my beers but not others. I’ve had dull malt flavors in some but not others. I’ve had muddled hop flavors in some but not others. And I’ve won a decent number of medals and generally consider my beer to be on the good end of the spectrum of homebrew with the odd obvious flaw mostly being clearly related to recipe issues, fermentation, or oxidation.
One if the very few things that ALL of these beers have had in common was a mash pH of 5.2-5.4 as calculated by bru’n water (so thats the room temp pH). So it would seem that it doesn’t really solve (or cause) any problems for me one way or the other.
Actually, I stopped paying much attention to targeting a specific mash pH, unless it is predicted to be totally whacked. I target the kettle pH, which always seems to involve a downward adjustment.
Targeting a kettle pH is also a whole lot easier than the mash pH (with regular brewing equipment that is).
Die_Beerery
Well-Known Member
- Joined
- Aug 21, 2017
- Messages
- 842
- Reaction score
- 643
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
What problem are you all trying to solve exactly by mashing at a higher pH?
Is it a purely academic exercise to match the intent of literature, or does it address specific issues in a tangible way?
I just want to brew tasty beer. The kind I brewed years ago before I started messing with lower end mash pH's. Time to return to the higher end. The commercial level peer reviewed literature I have delved into has assisted in guiding me back to the higher end. The key was unlocking the mash temperature pH vs. room temperature pH matter, and coming to the conclusion that 5.2 to 5.5 at mash temperature is the real "ideal" target. And further discovering that simulating 5.2 to 5.5 pH during the mash and as measured at mash temperature is as easy as measuring at room temperature and adding ~0.3 pH points, thus targeting 5.5 to 5.8. For which the midrange is 5.65. The exact same 5.65 pH that @Robert65 had mentioned awhile back (and on a different beer brewing forum) is his favorite mash pH target. With his reason being the same, I.E., that he started making the best beer he ever made once he started mashing at a target of 5.65 pH as measured at room temperature.
Last edited:
aeviaanah
Well-Known Member
- Joined
- Jul 1, 2012
- Messages
- 1,686
- Reaction score
- 217
Wow! I cannot believe that was not clear in my original post and later reply. That is exactly what I meant @Oginme.
Why so much confusion remains around such a straight forward concept is beyond me. I hope the following is clearer and easier to understand now.
“The pH meter is first calibrated with calibration solutions at 77F/25C for a sample taken at the same 77F/25C temperature.
The pH meter is then calibrated with calibration solutions at 152F/66.7C for a sample taken at the higher 152F/66.7C temperature.”
This isn’t confusing to me but rather interesting- I had no clue I should be calibrating my pH meter (with ATC) across various temp ranges using the same solution
I will say, for those of us without practical access to RO water, or an easy means to independently dose sparge water (a good representation of much of your smaller craft brewers), the plus side to starting acidification in the mash is that it keeps runoff pH down. I couldn't imagine mashing at 5.8, then running off and driving it up from there.
So a 20-ish C 5.4-5.5 mash, last wort around 5.7-5.8, kettle pH of 5.3 (or driven down to it), and knockout pH of about 5.1. That works for me, consistently.
Do what works and do it consistently.
Everything else is just a circle jerk.
So a 20-ish C 5.4-5.5 mash, last wort around 5.7-5.8, kettle pH of 5.3 (or driven down to it), and knockout pH of about 5.1. That works for me, consistently.
Do what works and do it consistently.
Everything else is just a circle jerk.
Last edited:
That's because you don't have to. That is what ATC is there for.This isn’t confusing to me but rather interesting- I had no clue I should be calibrating my pH meter (with ATC) across various temp ranges using the same solution
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
That's because you don't have to. That is what ATC is there for.
Precisely!
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
I will say, for those of us without practical access to RO water, or an easy means to independently dose sparge water (a good representation of much of your smaller craft brewers), the plus side to starting acidification in the mash is that it keeps runoff pH down. I couldn't imagine mashing at 5.8, then running off and driving it up from there.
I don't presently sparge, so I don't experience a rise in run-off pH as a consequence of sparging, but mitigating that issue via knocking out sparge water alkalinity to a trivial level via acidifying it to 5.4-5.5 pH has always been very good advice, as is the advice to never over-sparge.
Technically, the HCO3- bicarbonate species that causes most alkalinity fully ceases to exist in water at pH 4.5 [some say pH 4.3]. A single drop (0.1 mL) of 88% lactic acid added to 5 gallons of truly excellent RO water should bring it to the 4.3-4.5 pH range, or even potentially a tad lower, particularly if it is by some miracle fully alkalinity free to begin with, albeit that RO does indeed have a small level of extant alkalinity, and if high enough one drop of acid will not get you there. If you have concern that your sparge RO may not be of the highest quality, adding a single drop of lactic acid to it won't hurt anything (even if it does result in 4.3 pH) and will reduce extant alkalinity. The buffering capacity of the grist, even though it is continually being eroded away via sparging, will assure you that a single drop of lactic acid added to 5 gallons of RO sparge water will cause no harm. Short version: It can't hurt, but it may help.
Last edited:
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Arthur Schopenhauer, German PhilosopherAll truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident.
The key here is in discovering what is in fact truth, and what isn't. Sometimes to find it you have to climb out of the current box (safe space) of popular choice and experiment.
If pH adjustments are only important in the kettle then I guess there is no need to adjust mash pH. Is that what's being proposed here?
Apparently you're not alone @aeviaanah but the information supporting ATC and calibration process is out there from many sources.This isn’t confusing to me but rather interesting- I had no clue I should be calibrating my pH meter (with ATC) across various temp ranges using the same solution
"Temperature affects pH in a variety of ways – for a detailed look at them see this article, but overall we can say that the two most important things to remember are;
- Keep your samples and calibration buffers as close in temperature as possible
- Use a meter with ATC to compensate for the remaining small differences"
"ATC sounds great. Is it perfect? ATC is great, but is not perfect. ATC is based on theoretical assumptions for a perfect, ideal electrode; however, no electrode is perfectly ideal. The better the electrode, the better the ATC works. The farther apart the temperatures of the samples and calibration buffers are, the larger the correction and the more chance for introducing error due to non-ideal behavior. The chart below shows some testing conditions and how they compare for accuracy and convenience."
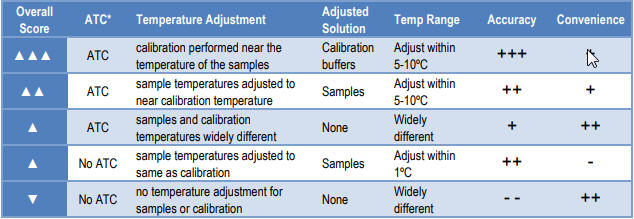
"The best accuracy occurs when a quality electrode (in good working condition) is used, ATC is used, and the sample temperature vs. buffer temperature is not drastically different. Note that ATC is much more accurate than not performing temperature compensation. It is more convenient that adjusting sample and buffer temperatures to the exact same temperature, and it is more convenient than manual temperature compensation."
Last edited:
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
If pH adjustments are only important in the kettle then I guess there is no need to adjust mash pH. Is that what's being proposed here?
Much depends upon ones recipe, mineralization, and mash water alkalinity. I'm (currently at least) only willing to initially target as high as pH 5.65 as measured at room temperature within the mash for my recipes. Anything projected to be higher than 5.75 and (currently) I would react (preferably pre-mash) to adjust it downward to 5.65 pH within the mash. Ditto for anything projected to be lower than 5.55 pH in the mash (as measured at room temp), for which case I would (currently) also react (preferably pre-mash) to raise it to the 5.65 target. Effectively, with allowance for meter calibration and meter precision tolerances**, this allows for 5.5 to 5.8 pH (measured at room temperature) as being fully acceptable within the mash, commensurate with the peer reviewed commercial brewing level literature.
**Many digital pH meters, despite being capable of displaying to 0.01, state within their actual specs that 0.02 to 0.05 is all they can actually guarantee as to precision.
Last edited:
Let's say I'm brewing a Pale Ale using a simple recipe of 2-row, Chrystal malt and 100% untreated RO strike water. Where the predicted pH is 5.57 would you mash as is and adjust only the pre-boil kettle pH @Silver_Is_Money?Much depends upon ones recipe and source water alkalinity. I'm (currently at least) only willing to target as high as pH 5.65 as measured at room temperature within the mash. Anything higher than that and (currently) I would react to adjust it within the mash.
Similar threads
- Replies
- 7
- Views
- 1K
- Replies
- 6
- Views
- 1K
- Replies
- 6
- Views
- 1K
- Replies
- 17
- Views
- 5K













































