hopjuice_71
Well-Known Member
- Joined
- Mar 26, 2016
- Messages
- 373
- Reaction score
- 374
They are asking for $50 to download it.
I was worried about that. The link takes me to a paywall as well. I just tried searching the whole title on Google Scholar (Factors Affecting Hop Bitter Acid Isomerization Kinetics in a Model Wort Boiling System) and it gave me a direct link to the pdf. Maybe try that?
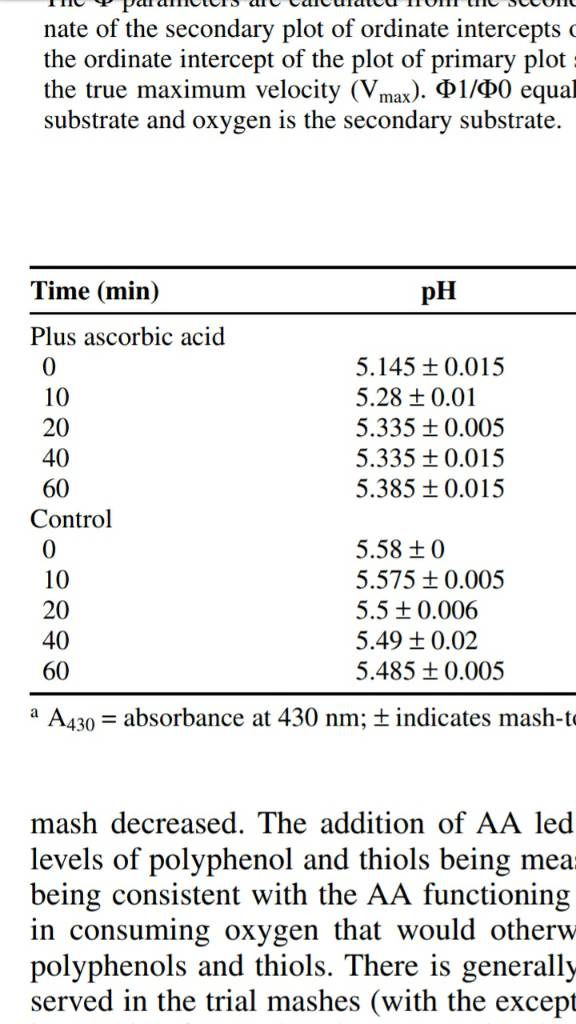
One of the main advances, I feel, is that the authors carefully consider the relationship of a-acid solubility with temperature and pH, which hadn't really been done before (as far as I can tell). a-acids are fully soluble (within limits) at boiling or near boiling temperatures; however, when samples are cooled, their solubility decreases and they precipitate. Solubility is also pH dependent, meaning more material will precipitate if at a different pH. So, when samples are taken for measurement of a-acid and iso-a-acid and cooled to measurement temperature (room temp), some of the a-acid won't be measured as it has precipitated, and done so in a pH dependent manner as well. Because of this issue, which is a technical flaw in the assay, it makes it appear that hop utilization is pH dependent, when it likely isn't.
Last edited:



![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)