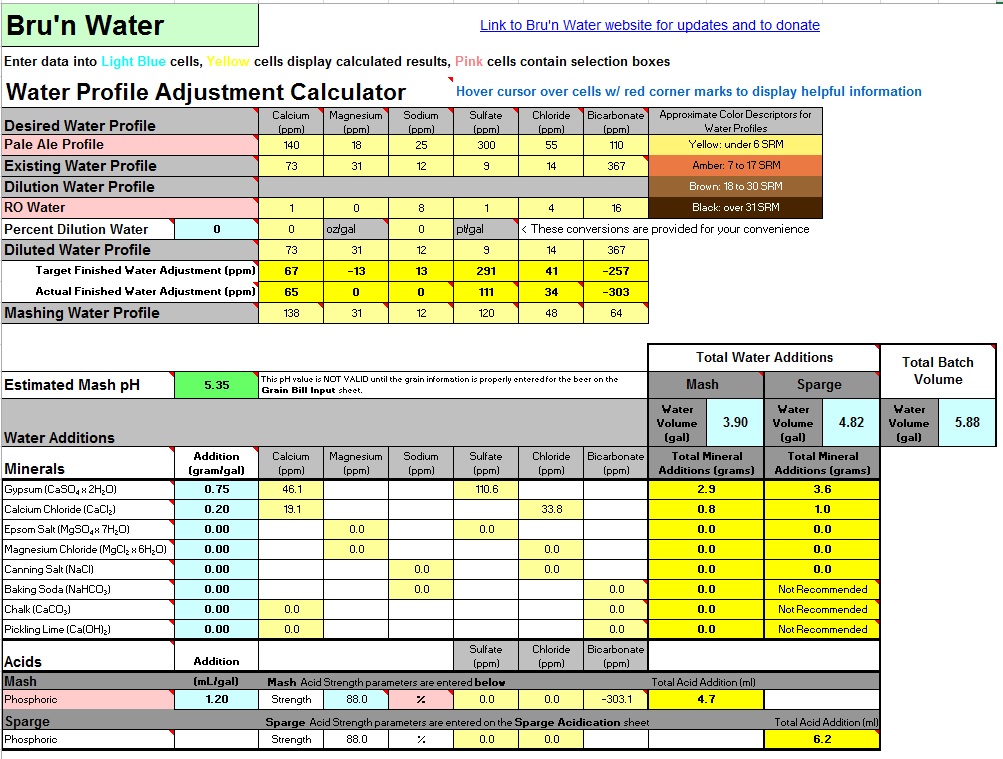
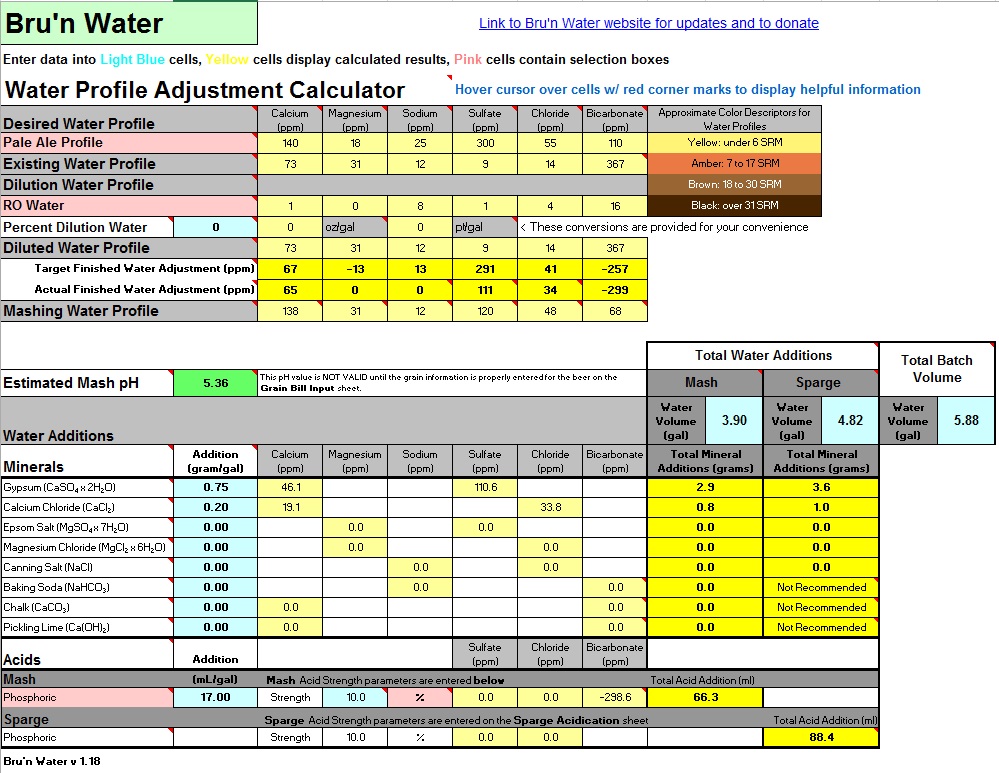
I got a water test done and have the information below. The last couple batches I have been adding lactic to the mash water per Brewcipher but I was thinking I might go further and dilute or add minerals as necessary for a Sierra Nevada pale ale clone I'm doing this weekend. I also use campden for chlorine. Any suggestions on what to do?
73 Ca. 31 Mg. 12 Na. 14 Cl. 9 SO4. 367 HCO3. 7.4 pH
Recipe is 9.67 lb 2 row. 0.73 lb crystal 60L. 3.9 gallon strike water
I downloaded Bru N Water today and it is telling me 7.5 mL of lactic acid and Brewcipher is saying 9 mL to get to 5.35-5.36 mash pH. Maybe I have been putting too much acid in as well.
73 Ca. 31 Mg. 12 Na. 14 Cl. 9 SO4. 367 HCO3. 7.4 pH
Recipe is 9.67 lb 2 row. 0.73 lb crystal 60L. 3.9 gallon strike water
I downloaded Bru N Water today and it is telling me 7.5 mL of lactic acid and Brewcipher is saying 9 mL to get to 5.35-5.36 mash pH. Maybe I have been putting too much acid in as well.






































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)