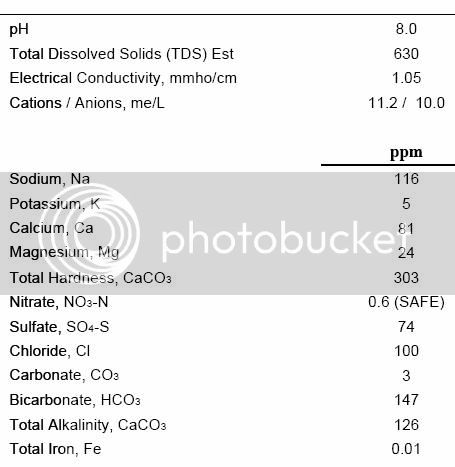
Revy: your water is very close to mine.
I just spent way toooo much at brewsmith water profile. Plug your numbers into base water(need to be in PPM, not mg/l). Palmer has a conversion chart if you need to change them over. Also on his page is a list of each mineral and its affects on beers including amounts affecting sweetness, high/low bittering, dark/amber/light beers. After you plug in your base water values; select target(essentially a desired city water) and it will show you the differences between the 2 water profiles. I am making a nut brown ale and choose London. I compared its profile with Palmers information. Seems Londons water is close to what I want for a NBA. After both profiles are entered I adjusted the mineral additions(on left side of page). As I adjusted a mineral I watch the differences change. Still playing w/ that.
Ultimately the thing to do is create your own target water, say stout, enter the needed mineral concentrations and compare to your own profile, then add minerals. I am surprised Brewsmith does not do this. Seems simple to do.
conpewter: There is also a section on brewsmith for dilution. So you enter your water profile, dilute with(for you I am thinking distilled) and enter quantity, then pick desired target profile. The higher CO3 you are the more you dilute. If you don't have brewsmith download a free version for 30 days.
Enough about all of this for now....to be continued Charlie
Thanks Charlie, a lot for this info....Guess I gotta start pluggin numbers.









![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)