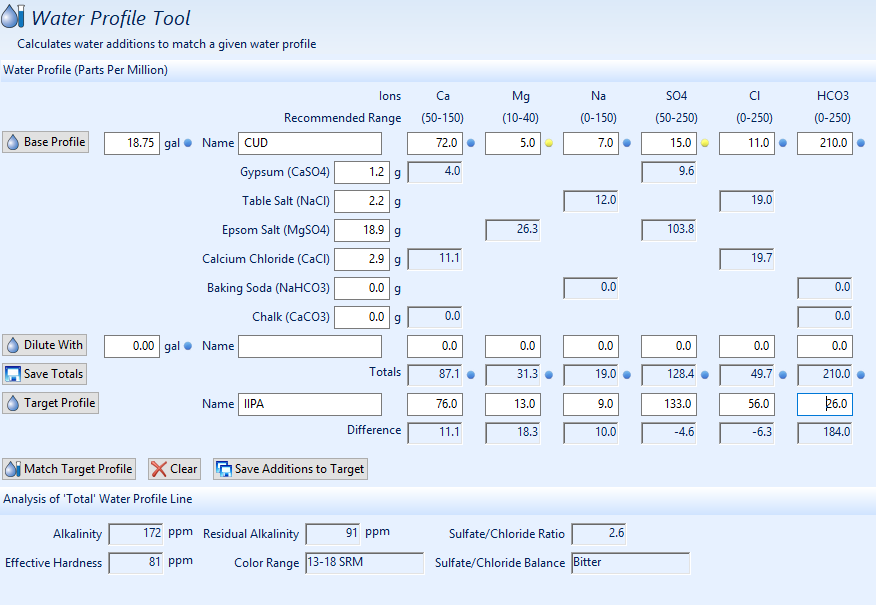
I think I have my water profile situation figured out, but this leads me to another question regarding water profile and sparge water. I have a on demand water heater that I use to fill my mash tun and fly sparge with. Given that this is my method of brewing, I'm not able to treat my sparge water prior to sparging. So, looks like there are 3 possible options if I don't want to change my mash/sparge method.
What is the best thing to do in this situation? Any other options?
Thanks!
- Treat mash water only with proper additions for that amount of sparge water: ie it calls for 18.75 gallons of water to sparge with, but the total water needed is 35.12. Only treat the 18.75 gallons. Leave out the rest.
- Treat mash water with ALL additions for the total amount of water: ie: treat the 18.75 gallons of water as if it's 35.12 gallons.
- Splt it up. Treat mash water with proper additions for 18.75 gallons and then add the other additions directly to the boil.
What is the best thing to do in this situation? Any other options?
Thanks!







































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)


















