GreenMonti
Well-Known Member
- Joined
- Nov 29, 2009
- Messages
- 1,268
- Reaction score
- 67
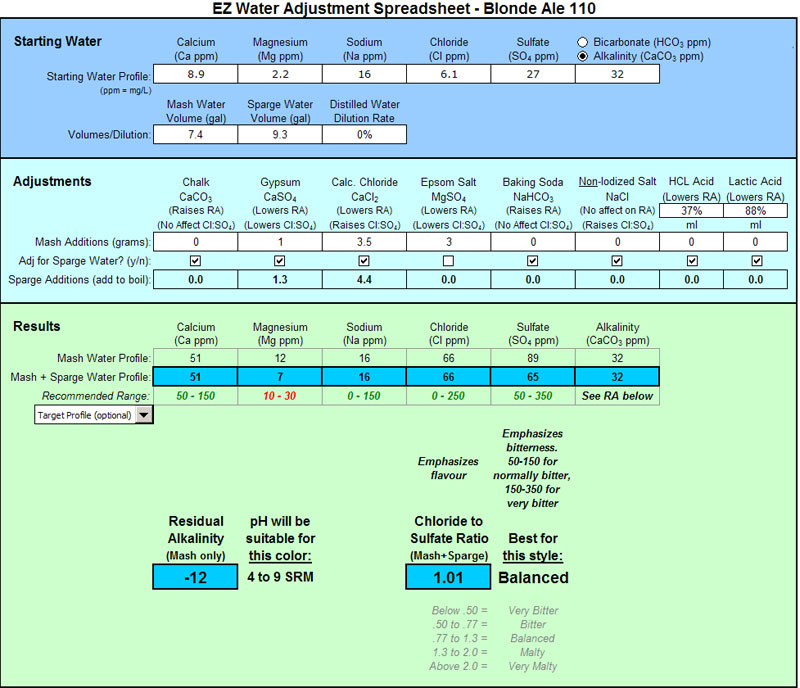
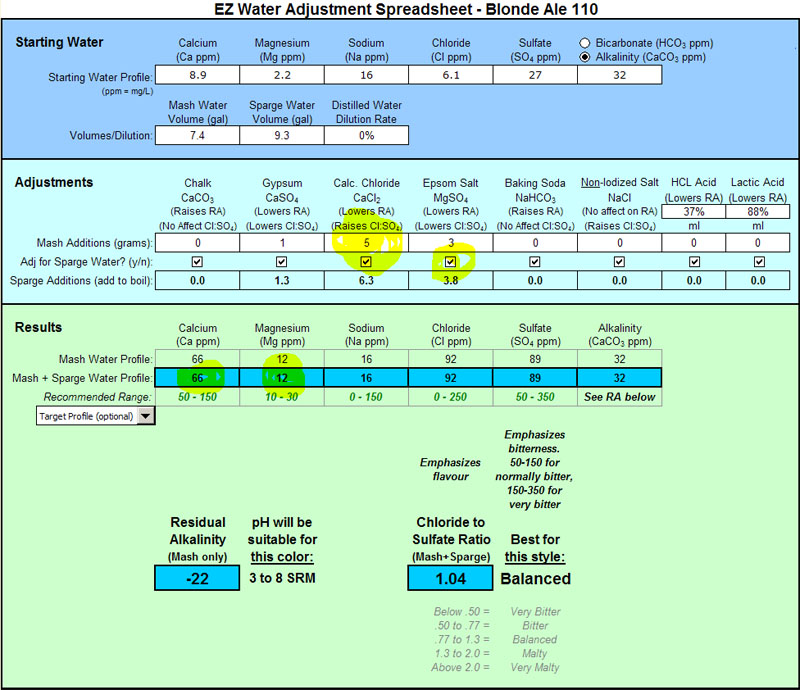
Nice work Bobby_M. Thank you for putting this together. I am just getting interested in water profiles. This helps out a lot.
This is just a thought.
I know that municiple water is different at times. What you get depends on what they were supplying that day. I don't know how different the water is or what it is that they change. Does anybody know what I am talking about? If so, are the changes big enough to even matter?
Sorry if this was asked or adressed eairlier on. I didn't read the whole thing.
This is just a thought.
I know that municiple water is different at times. What you get depends on what they were supplying that day. I don't know how different the water is or what it is that they change. Does anybody know what I am talking about? If so, are the changes big enough to even matter?
Sorry if this was asked or adressed eairlier on. I didn't read the whole thing.
















































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)