seagondollar
Well-Known Member
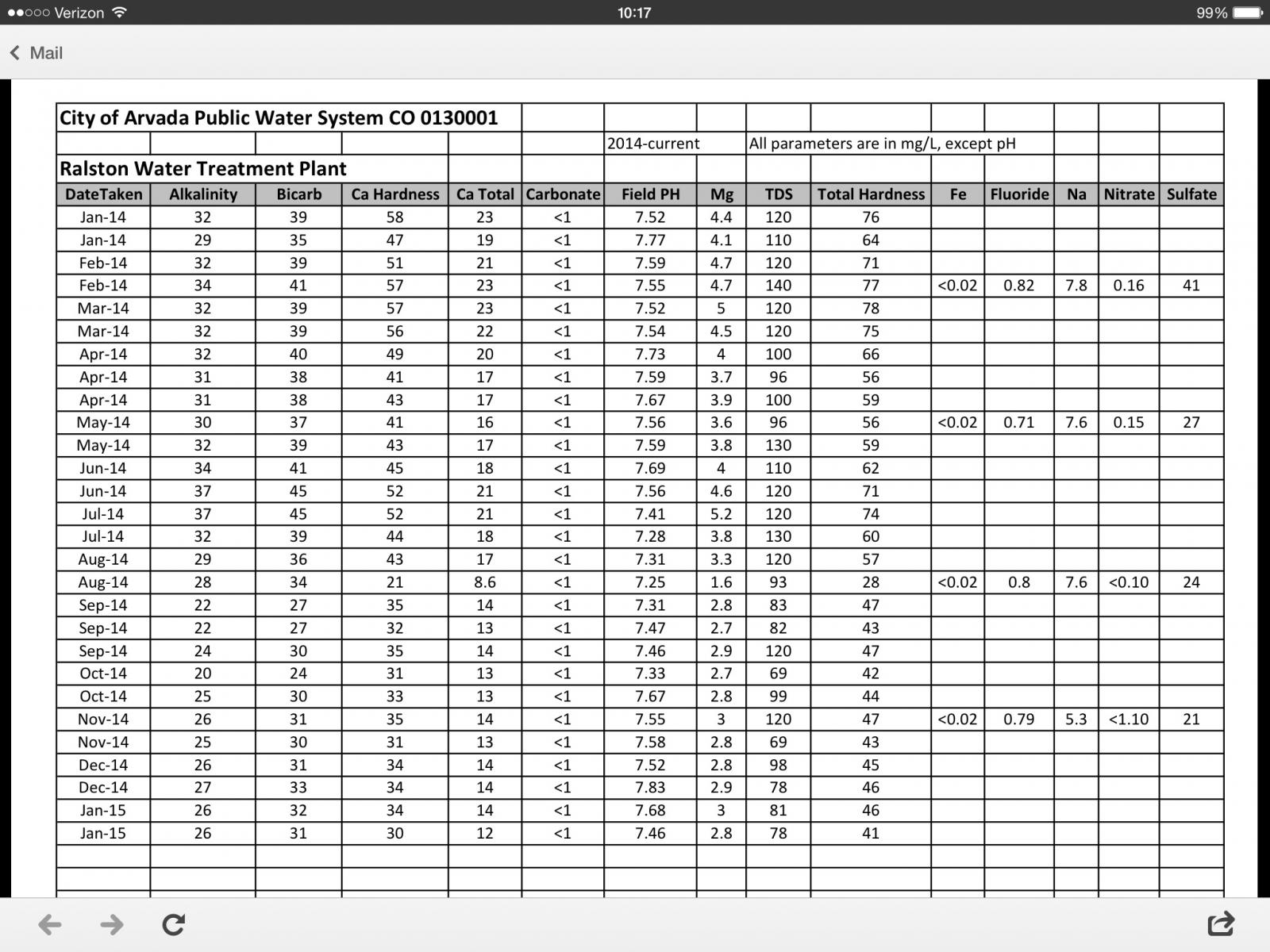
Ive been diving into the water characteristics and beer lately, because my pale ales seem to be lacking (SRM less than 8 id say). Im in arvada colorado and according to brewers friend, my water is as follows:
Ca: 50ppm
Mg: 1ppm
Na: 16ppm
Cl: 12ppm
So4: 38ppm
Alkalinity: 40
Ph 7.6
With that in mind, when i got my water quality report last week from arvada water everything was generally the same except for the calcium totals. It lists calcium hardness as 30 and calcium total as 12. Does anybody know what the difference is there? Or what number goes in my water software for calcium totals? Might be a dumb question, be gentle.
Ca: 50ppm
Mg: 1ppm
Na: 16ppm
Cl: 12ppm
So4: 38ppm
Alkalinity: 40
Ph 7.6
With that in mind, when i got my water quality report last week from arvada water everything was generally the same except for the calcium totals. It lists calcium hardness as 30 and calcium total as 12. Does anybody know what the difference is there? Or what number goes in my water software for calcium totals? Might be a dumb question, be gentle.



