- Joined
- May 6, 2025
- Messages
- 303
- Reaction score
- 238
Hello all
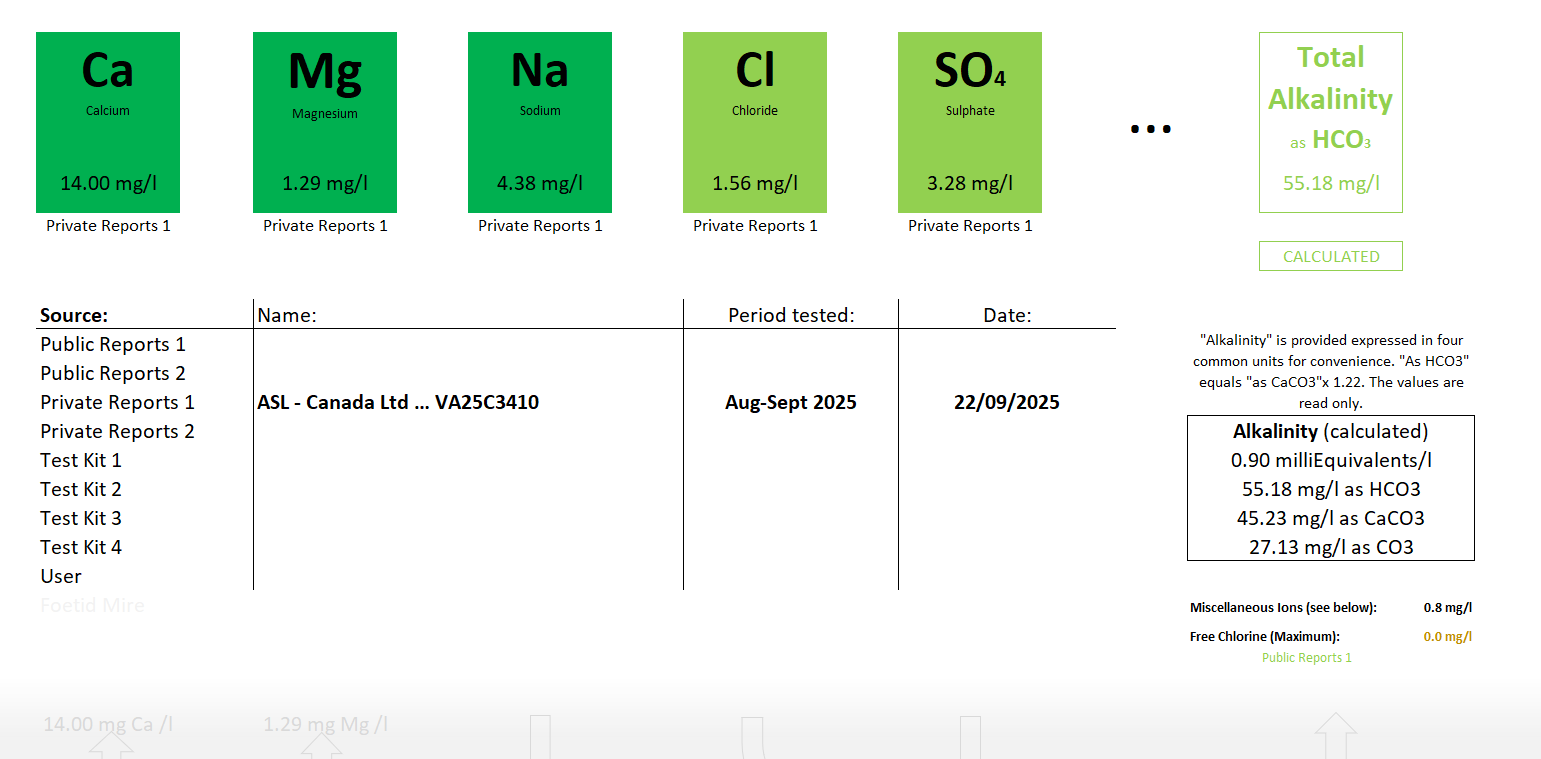
I am attaching a laboratory water analysis that I commissioned. It is untreated ground water taken from a Gulf Island on the west coast of Canada. Our club has an oceanfront camping area. River water runs underground, through the "fines" and we collect it. It is untreated. The river waters are in unoccupied forest lands. Nobody has become sick with e coli from drinking it.
I recently took five gallons home. And I sent a liter to a private laboratory for analysis to be used in brewing. I instructed the lab to test for the minerals that are relevant in beer brewing.
I currently have the ingredients to make a three gallon batch of Pale Ale, using this water.
I am new to brew water science. I am just starting to read about it.
If anybody can look at the lab results and give me some helpful suggestions that would be appreciated.
At home, my city tap water is soft. I have a reverse osmosis machine. I have bags of calcium chloride and calcium sulphate.
Maybe this untreated water is fine the way it is. Or maybe I need to add some RO water to lighten it up. Or maybe I need to add minerals.
Your thoughts are appreciated. Again the lab results are attached
I am attaching a laboratory water analysis that I commissioned. It is untreated ground water taken from a Gulf Island on the west coast of Canada. Our club has an oceanfront camping area. River water runs underground, through the "fines" and we collect it. It is untreated. The river waters are in unoccupied forest lands. Nobody has become sick with e coli from drinking it.
I recently took five gallons home. And I sent a liter to a private laboratory for analysis to be used in brewing. I instructed the lab to test for the minerals that are relevant in beer brewing.
I currently have the ingredients to make a three gallon batch of Pale Ale, using this water.
I am new to brew water science. I am just starting to read about it.
If anybody can look at the lab results and give me some helpful suggestions that would be appreciated.
At home, my city tap water is soft. I have a reverse osmosis machine. I have bags of calcium chloride and calcium sulphate.
Maybe this untreated water is fine the way it is. Or maybe I need to add some RO water to lighten it up. Or maybe I need to add minerals.
Your thoughts are appreciated. Again the lab results are attached






















![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)