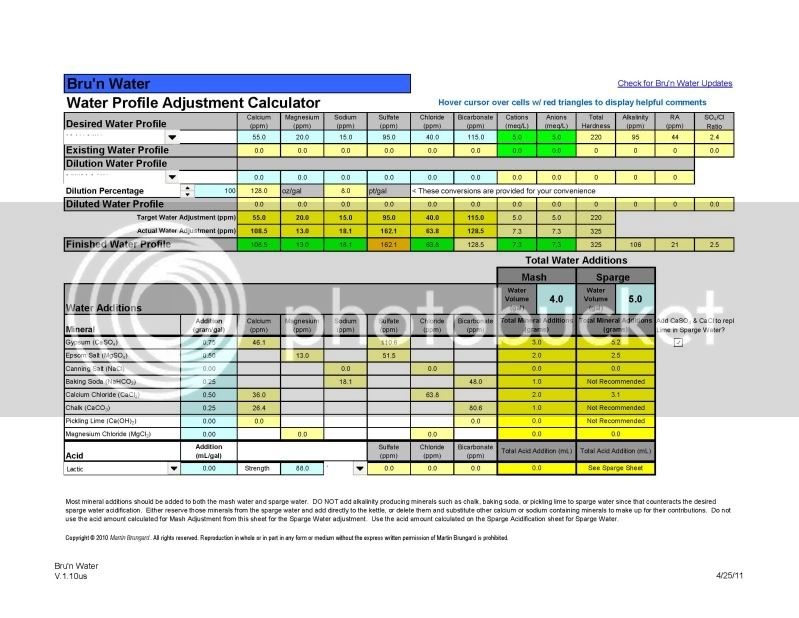
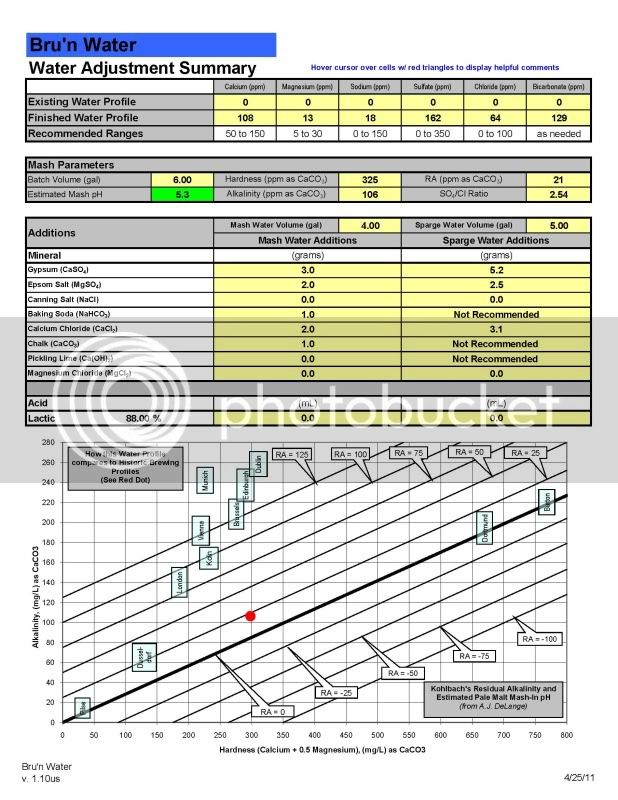
I'm thinking now that I'll just start out reconstituting my RO water to a particular balanced water profile and tweak it as I learn.
At the risk of getting you even more confused the issue here is really the variability of the malts. The water is, by comparison, quite simple and, within certain bounds (outside of which home brewers seldom venture), well understood. The bottom line is that you can't predict mash pH with a rule of thumb and you can't predict it with a spreadsheet unless you have measured the titratable acidity of every malt in the grist. As that's not practical, the spreadsheets must model, for example, 60L caramel malt as having a certain acidity. Some lots from some harvests from some maltsters will behave close to the way the models predict and some won't. And this pertains to base malts as well as specialty malts. I've measured Maris Otter base only DI mash pH as low as 5.6 and I've measured 5.7 in the mash tun in a grist using 95% Maris Otter and 5% 60L crystal which implies that that batch of MO had a much higher DI water pH. Thus Martin's statement that you will definitely need alkalinity isn't necessarily true though it represents a reasonable prediction given that you are using a lot of 60L crystal.
You can only guess whether a particular rule of thumb or spreadsheet (I haven't seen one that asks about base malt DI water mash pH) will get you to the mash pH you desire and in case you haven't figured out where this is going yet, it's a pitch to sacrifice some other aspect of life (if you have a life outside brewing) and get a pH meter. This is the only way to be sure what is actually happening. By the use of a pH meter you effectively measure the titratable acidity of the grist components in the matrix in which they are to be employed but do it implicitly i.e. no acidity calculation is necessary - you get the mash pH value and that's what you are after.
I downloaded brunwater but it is quite a bit over my head right now. I have read quite a few good reviews of it though and some day I'm sure it will be a valuable tool for me.
You don't set out learning how to play the piano by tacking the Goldberg Variations and you don't jump into brewing water chemistry at the same level as Charlie Bamforth. What the spreadsheets can teach you is what the relationships between alkalinity, pH, malt acidity, added acids, dilution water etc. are for example if I have a liter water at pH 8.3 with a given alkalinity and hardness (and sulfate....)and add x grams of 2% sauermalz and a liter of a different (RO or spring) water with a lower alkalinity and hardness (and other mineral composition) to it what will the pH of the new water be and what is the likely effect on mash tun pH. This is very instructive. Where it falls down is that the Sauermalz I am using may have acid content anywhere from 1 - 3% (2% is sort of an average) and if I haven't specified the titratable acidity of the base (and other malts) I cannot accurately predict mash pH. But you can learn the relationship between, in this example, dilution water, sauermalz and mash pH.
All the comments about spreadsheets go, of course, in spades for the rule of thumb approach as well. The primer is based on hours with spreadsheets, hours in the lab and hours in the brewery. But it's still a bunch of rules of thumb.
My assumption has been that most brewers don't measure pH.
That's true and it includes professional ones. I even gave a meter to the local gastropub guy because he brews the some of the same beers I do with essentially the same water and ignores pH. I know his beers could be better if he used enough sauermalz to get the pH into the "correct" range but he wasn't taught that way. OTOH the local Gordon Biersch (also using the same water) does control mash pH (with sauermalz)
Are non-ph-measuring brewers just not making as good a beer as the recipe is capable of producing?
That, in a nutshell, is it exactly.
Some of the earliest spreadsheets advised such absurd levels of added alkalinity that beers produced with them must have been (and have been reported to me as being) pretty bad but the newer spreadsheets, such as Bru'n Water, have toned that down considerably.
Despite my stubborn refusal to add alkali to my beers I've never experienced a mash pH that was too low but then I would never use as much as 21% 60L crystal in a beer either so I can't comment on what happens if mash pH is too low. When people started following the primer recommendations (and thus lowering what had been high mash pH's into the proper range) I started getting reports back to the effect that "all the flavors are brighter" and I really think that says it best.
































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)