Just out of curiosity, how many high gravity batches have you brewed using the olive oil method and what were the results?
Using olive oil at the level that I brew these days is impractical because the amount needed is so small that it would be difficult to measure (I currently brew 1 to 3-gallon batches).
The point that I was trying to make is that yeast cells do not respire in the presence of glucose levels higher than the Crabtree threshold (which all beer worts exceed). The reason yeast cells need oxygen at the beginning of fermentation is for sterol and unsaturated fatty acid (UFA) biosynthesis. If the yeast culture pitched into one's wort has adequate stores of these compounds and the number cells pitched is large enough that maximum cell density is only a couple of generations away from the mother cells, then dissolved oxygen requirements drop radically (the reason why properly pitched dry yeast does not require aeration). If we supply sterols and UFAs directly to the yeast, the need to aerate goes away. This information is backed up by peer-reviewed science, as well as by an experiment that was performed at White Labs (
http://www.whitelabs.com/blog/olive-oil-vs-aeration-experiment).
We need to remember that fermentation is an anaerobic process (known formally as anaerobic glycolysis). All cell reproduction in normal and high gravity worts is anaerobic. The ability of a yeast culture to reach terminal gravity in a high gravity wort is a function of the sterol and UFA reserves found in the mother cells and how close to maximum cell density a culture is when pitched. The mother cells share their sterol and UFA reserves with all of their offspring. As sterols and UFA reserves fall, yeast cell membranes become less permeable. In essence, yeast cells do not stop fermenting because there is not enough dissolved oxygen. Yeast cells stop fermenting because they can no longer pass nutrients and waste products through their cell walls. It's the same reason why yeast cells stop fermenting above a certain alcohol level. However, in that case, the cells lose their ability to pass nutrients and waste products through their cell walls because they become dehydrated due to the fact that ethanol is highly hygroscopic.
Needing to aerate wort with pure oxygen in order to reach terminal gravity is a sign that one is pitching too little yeast. The oxygen that is not taken up by the mother cells is taken up by the daughter cells in order to support greater numbers of divisions. I do not have attenuation problems. I have over-attenuation problems because I pitch a large number of healthy cells into my beers. Most of my beers run 7%+ ABV, which isn't exactly low gravity beer. Other than the miniscule amount of oxygen that my wort picks up while draining from my kettle into one of my primary fermentation vessels, I do not aerate my wort at all. All I do is pitch a yeast culture that was grown under aerobic conditions; therefore, the culture is very healthy going into the primary.
I have included a few yeast-related photos from my brewery for those who may believe that I am here to troll HBT. The reason why I came to this forum in the first place is because it appears to be epicenter of the yeast rinsing movement on the Internet. Those who have read my postings know I how I feel about the voodoo-laden process of rinsing yeast with boiled tap water.
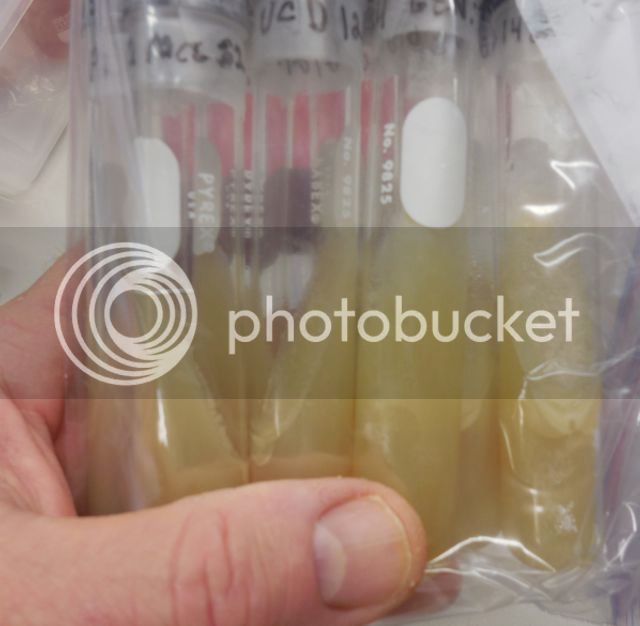
My Current Culture Collection (none of the cultures are Wyeast or White Labs cultures)
Cultures that I Isolated (Plated) from Brewery Sources
The culture tube with "HAR" on the Parafilm in the photo shown above is Harpoon's yeast culture. The "GEN:1" on some of slants denotes that a slant was inoculated directly from a plated cultured (I am in the process of rebuilding my bank after a long hiatus from the hobby). The number to right of the colon is incremented every time I subculture a slant to another blank slant (i.e., the next subculture will be GEN:2). This number is not a yeast generation number. It is a culture generation number. Each slant was inoculated with one or more yeast colonies from a plate, each of which was the offspring of a single yeast cell.
Culture Collection Cultures
The cultures shown above were acquired from major research-oriented culture collections. These yeast cultures originally cost between $71.00 and $300.00 each. None are available from Wyeast, White Labs, or any other commercial yeast source. I like to purchase cultures from collections on solid media (i.e., plates or slants). However, some collections only supply yeast in lyophilized form in glass ampules. A lyophilized culture looks like a white spec of dust.
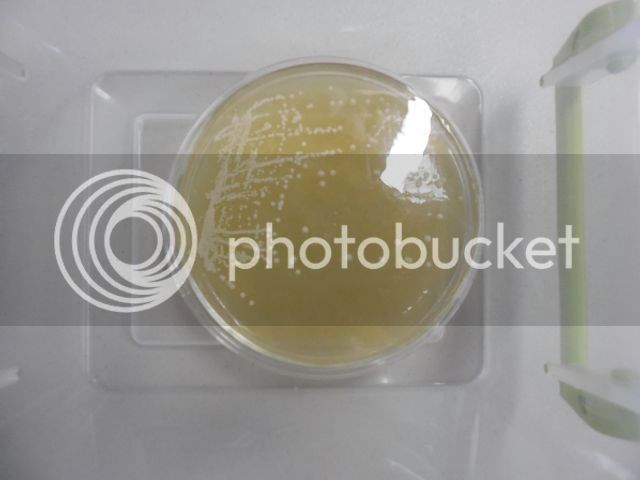
A Plated Yeast Culture
The plate shown above contains Scottish and Newcastle's Tyneside culture. The well-isolated round colonies are all each the offspring of a single yeast cell; therefore, they are single-cell isolates. Single-cell isolates are also known as pure cultures (Emil Hansen pioneered single-cell isolation at Carlsberg Laboratory). I streaked this plate a few days ago. Only well-isolated colonies that exhibit good morphology will be used to inoculate slants using aseptic transfer technique (one colony per slant for this culture).
40ml Sterile Starters
The container shown above contains 100ml media bottles that each contain 40mls of absolutely sterile wort. The 40mls of media in each bottle was autoclaved in the bottle. I use these bottles when propagating a yeast culture from a slant. Using absolutely sterile wort to propagate yeast from a slant allows me to start a culture using only a loopful or two of yeast without fear of infection.
A 40ml Sterile Starter
The bubbles in the starter shown above are from moving it. The media is 100% sterile because it was autoclaved for 15 minutes at temperature and pressure levels necessary to achieve sterilization. The stripes on the autoclave tape shown below indicate that these levels were achieved inside of my pressure cooker. This piece of autoclave tape basically looked like plain old masking tape before the media bottles were placed into my pressure cooker (it was attached to one of the bottles).
In closing, while most brewers use yeast to brew, brewing is something that I do to get to play with yeast. Playing with brewer's yeast is what kept me heavily engaged in the hobby for the decade spanning early 1993 through early 2003, and it's what brought me back to the hobby after a ten and a half year hiatus.










![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)


















































