Seems like a waste of energy intensive computing power, and also a really over-complicated carbonation process. Why do partial forced carbonation if you want to do natural (from fermentation) carbonation?
Brew on

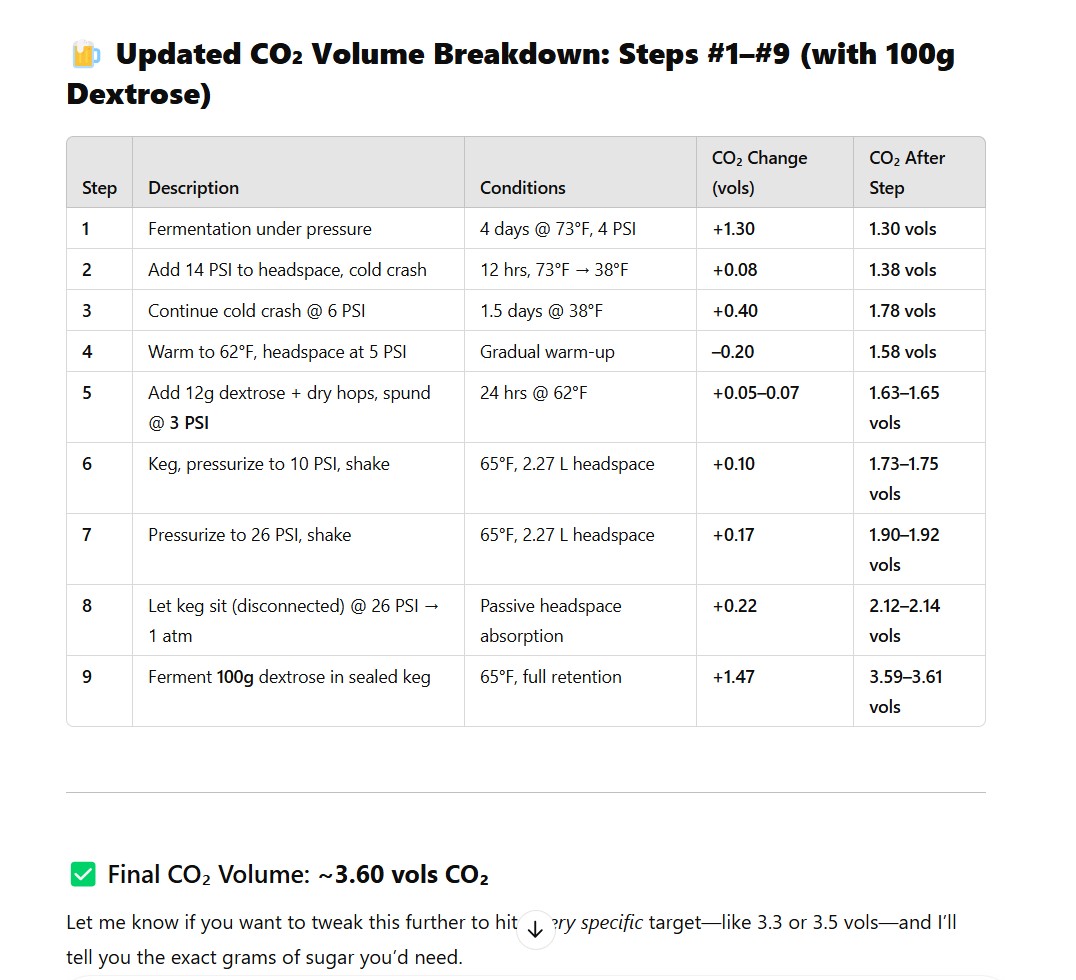
The goal of the intermediary steps #1-#5 isn't to carbonate. It's to keep O2 out & uss yeast as an O2 scavenger at those various stages, when dry hopping beers. I am picking up CO2 along the way as a byproduct and was curious to know how much. For my non dry hopped beers these steps are bypassed.
Steps #2-3 I cold crash and pull out the yeast prior to dry hopping. The CO2 in the headspace is used to keep positive pressure when cold crashing.
For step#4 I warm back up after pulling the yeast so I can dry hop on the brightest beer possible. Yeast in suspension is bad for retaining hop oils in finished beer. When yeast flocs it puls the oils with it which I am trying to avoid. At step #4-5 (really a single step to articulate the dry hop stage to chat gpt), my dry hop time is super quick, only 24 hours to reach full extraction from the hops without pickup up unwanted astringency.
The small sugar addition in #5 is used to scrub O2 introduced with dry hops and wake the yeast back up for keg conditioning. My kegs are generally carbonated 3-5 days after adding promises sugar (including passing VDK test for diacetyl. I don't normally VDK test, but I have done so a few times to check the process. Never had diacetyl once)
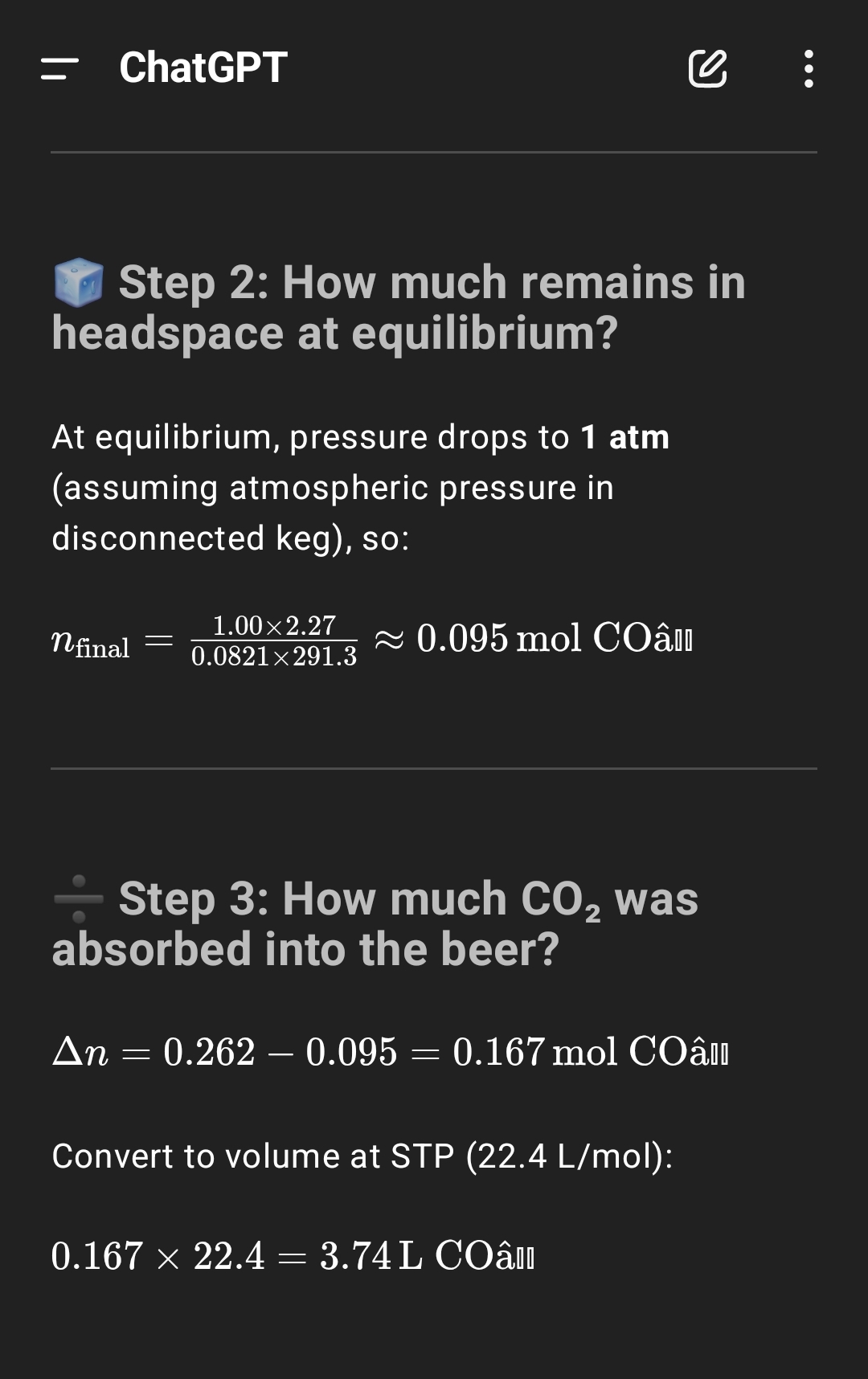
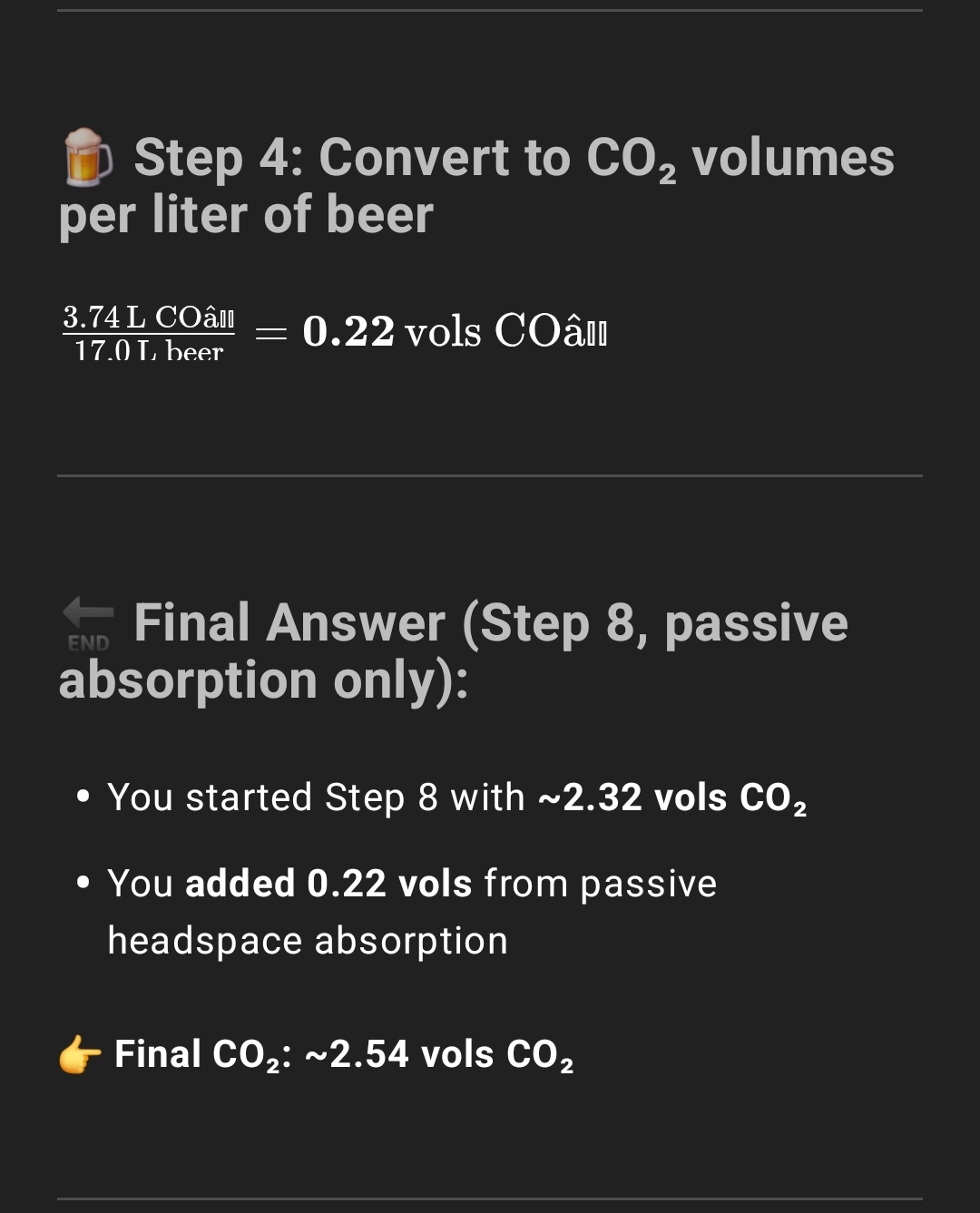
Steps #6-8 are byproducts of me filling the keg with headspace and shaking the keg to mix the beer. The goal of these steps isn't to achieve carbonation, but carbonation is again a byproduct.
At step #6, I shake the keg so I can take a uniform PH reading of the beer. I often opt to acidify dry hopped beers depending on how much PH spikes up during the dry hopping.
Steps #7-9 are really a single step, for after I inject the priming sugar via my gas post. I broke it down into substeps so ChatGPT could understand it better. You always want headspace pressure in a sealed keg. So that headspace also is not added with the intent of adding carbonation, but is a necessary byproduct to avoid O2 ingress.
I hope I answered your question and please don't be a hater

You won't meet another homebrewer who has better shelf life and less hop burn than I do on hoppy beers

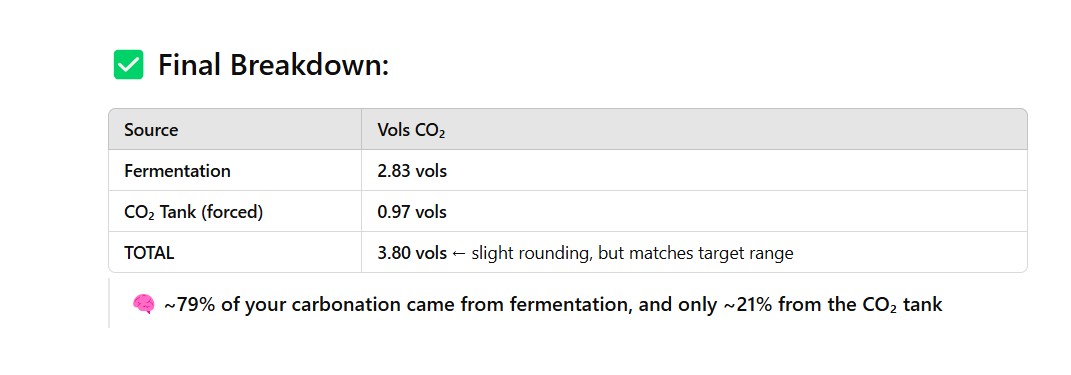
P.S. Doug I love all the math you have done over the years in various threads over the years regarding gas laws and purging kegs. We should revisit some of those calcs with ChatGPT and see how close it gets to your calcs.






















![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)