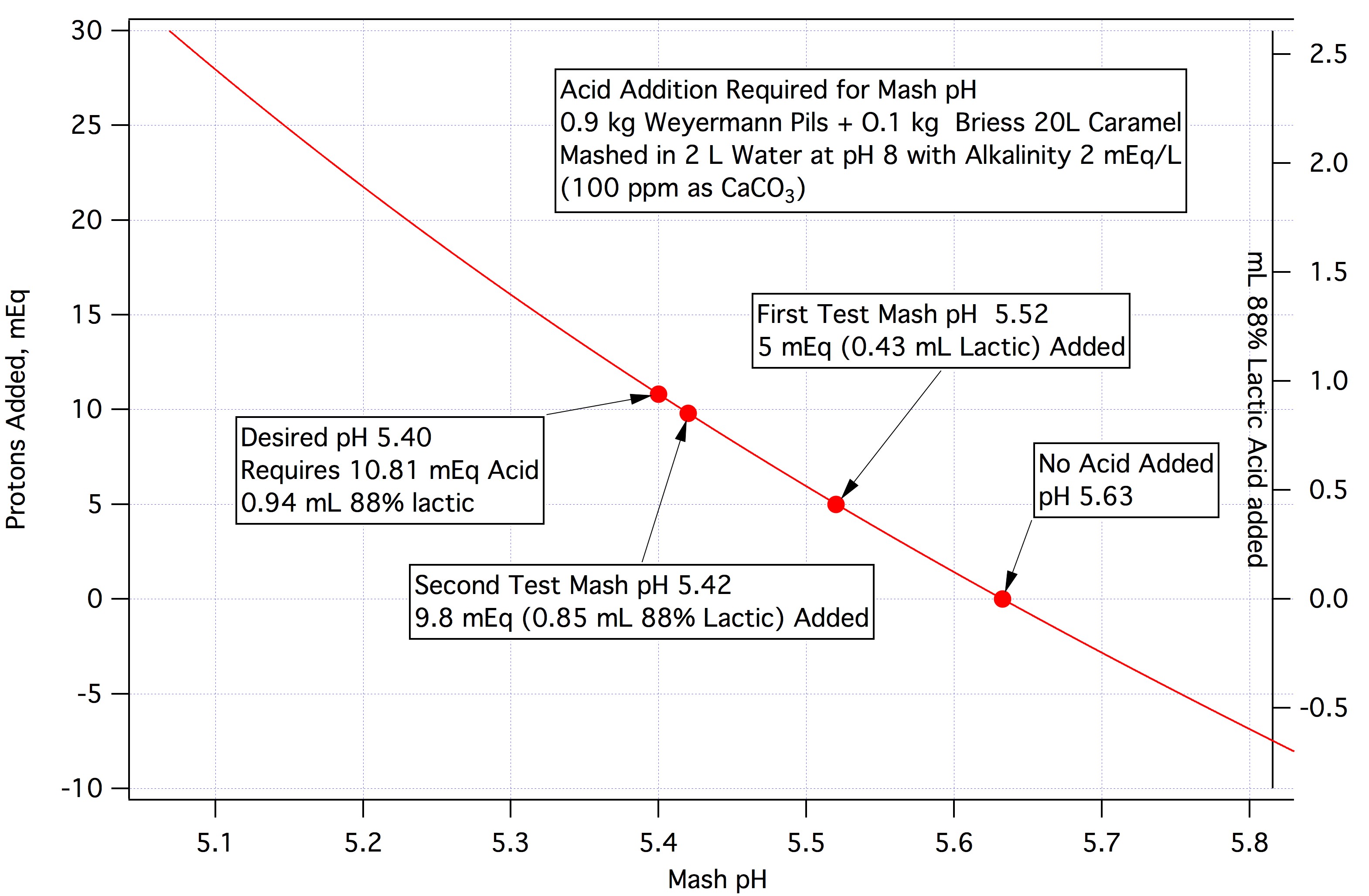
Ok, think I've got it. Keep me honest here. In the example above in wanting to move from 5.63 to 5.7. .07*40 = 2.8 mEq/kg protons to be removed. Since 1 mmol sodium bicarb absorbs 1 mEq protons, then 2.8*84=235.2 mg.
Check.
Just hadn't thought through. As usual when the light goes off, you sometimes wonder how you could have missed.
That's how the mind works. Sometimes, though, it is using the new concept that causes the light to come on. If you actually use the metric system, which people don't seem to want to adopt, eventually you will realize that if the sign says it's 444 km to Ayers Rock it will take you 4.44 hrs to get there if you drive 100 km/hr and it a lot easier to multiply the .44 by 60 to get 28 minutes for the fraction than it is to look at 277 miles which, at 60 mph wiil take you 277 minutes and mentally compute 4:27 from that.
On a somewhat different topic, I'm curious that John Palmers water calculator doesn't even consider pH.
In another thread (
https://www.homebrewtalk.com/forum/threads/got-my-water-tested-now-what.647463/) I say
Thus we can say (and often do) that if the water pH be between 6.9 and 9.4 the acid requirement is 90% ± 2% of the alkalinity.
and show why this is the case.
Most of the calculators approach the problem by reasoning that most of alkalinity is bicarbonate in the pH range in which brewers seem to operate. Fifty ppm of alkalinity implies 50/50 = 1 mEq of protons are being absorbed and as 1 mmol bicarbonate will do that in this pH range, the authors assume that 1 mEq of alkalinity is equal to 1 mmol of bicarbonate (61 mg/L). Thus if you have 50 ppm alkalinity they make you convert that to bicarbonate (61 mg/L) and have you enter that into their program. Then that they then convert to an acid requirement which is, as long as mash pH is reasonable and water pH within a certain range, going to be pretty close to 90% of the alkalinity and thus they do not need to consider either mash pH or original water pH in calculating that requirement. Because of the location of the first pK of carbonic acid (6.38) one can get away with this. A robust calculation, such as the one described in MBAA TQ vol. 52, no. 1, 2015, pp. 3-12 Predicting and Controlling Mash pH Using Simple Models for Mash Component Acid/Base Characteristics does, of course, take both mash pH and liquor pH into account. The algorithm defined there computes the protons necessary to be added or removed from each mash component in order to compute a total 'proton deficit' for a given pH. The estimated pH of a mash is the one that causes the total proton deficit to be 0. The total can be written as
Total = deficit_Malt1 + deficit_alkalinity + deficit_water +deficit_Calcium + deficit_Magnesium + deficit_acid + deficit_base...
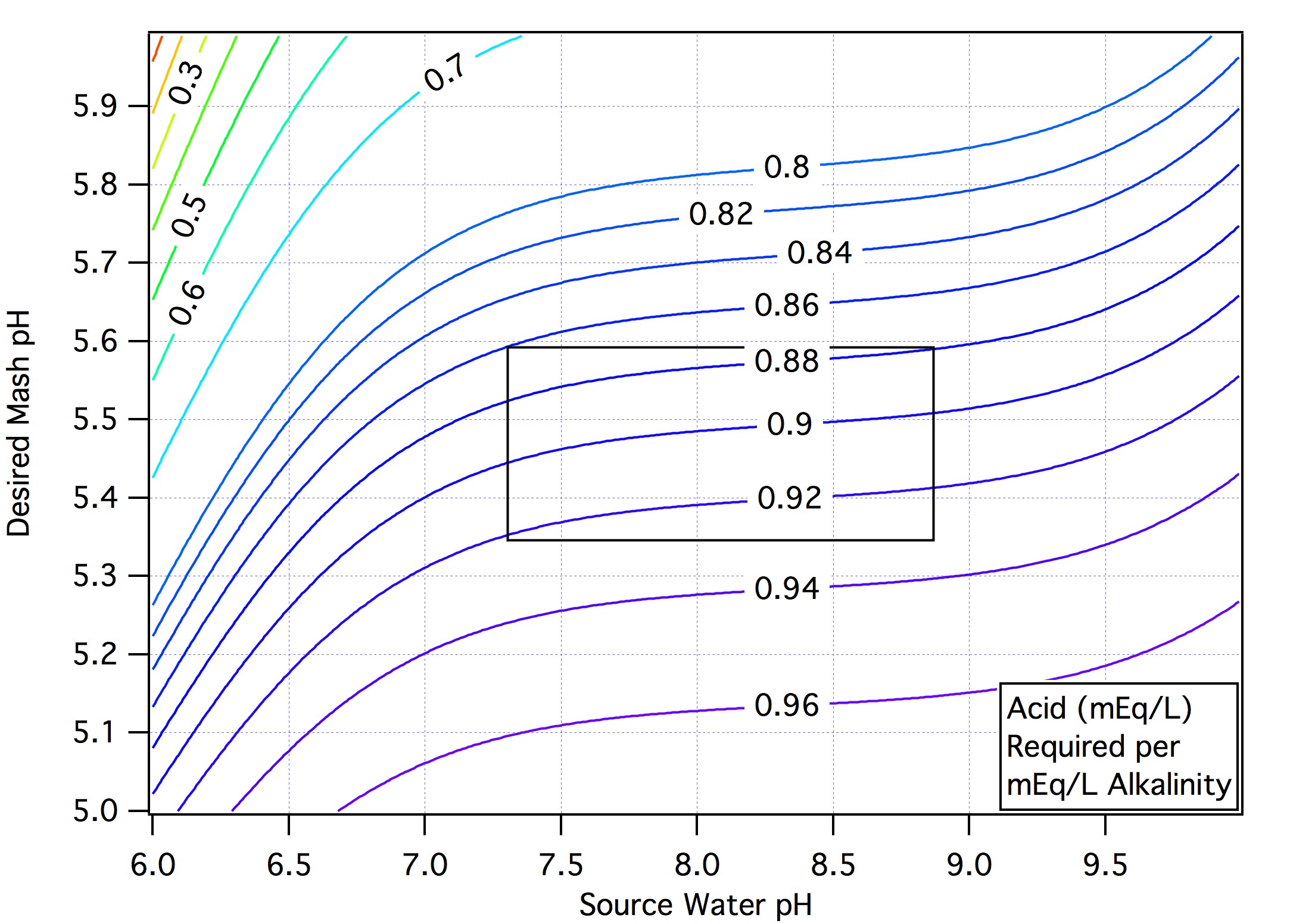
Base malts, bases and alkaline water (contains the base bicarbonate) absorb protons and have positive deficits. Calcium, magesium and acids supply protons and have negative deficits. All the terms in that sum except the calcium and magnesium terms depend on the mash target pH (and they well may too but at this point they are considered to depend only on the concentration of the ions) as does the alkalinity deficit but it depends on the source water pH too but the dependence is weak as following graph shows.
The alkalinity and metal ion sum is thus seen to be, in the boxed region of the graph:
deficit_alkalinity + deficit_Calcium + deficit_Magnesium ≈ 0.9*alkalinity+ deficit_Calcium + deficit_Magnesium ≈ alkalinity + deficit_Calcium + deficit_Magnesium = RA
The formal definition of RA is
RA = alkalinity - [Ca++]/3.5 - [Mg++]/7
Thus the robust calculation includes something close to the RA, i.e. 0.9*RA but not the RA as it is usually defined.
He is only interested in RA.
When John set out to write his book he was convinced that RA was the holy grail of mash pH prediction. I tried hard to convince him that it wasn't and in fact developed the robust algorithm to try to convince him of this. Obviously he accepted it at least partially as some of it appears in the book but he never fully let go of RA.
The little experience I have with the other water calculators is that pH is of the greatest importance.
I don't have much experience with any of them but none of them, that I can think of, ask for source water pH.
Just look at the various water profiles in Martin's program. Many end up with negative RA. Any thoughts?
The problem with Martin's profiles is that he wants certain cations and winds up with the levels of chloride and sulfate he wants with an excess of cations. So what is he to do? I don't really have any bright ideas other than to accept less calcium and magnesium or more sulfate and/or chloride be he has adopted the approach of adding bicarbonate. The problem with that is that bicarbonate's charge depends on pH. Martin decided to use the charge at pH 8.3. As bicarbonate comes to us at pH 8.3 one doesn't have to do any charge shift calculations if he assumes the pH is 8.3. The problem is that if you add bicarbonate to water the pH doesn't always come to 8.3. The short and long of it is that you can't make many of his profiles except at pH 8.3 and I believe his general advice with regard to this is to ignore the bicarbonate column.