I notice several entries call for lactic acid and sodium bicarbonate. Are you trying to get the sodium up without increasing alkalinity?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Stout Brewing Water
- Thread starter Stand
- Start date

Help Support Homebrew Talk:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
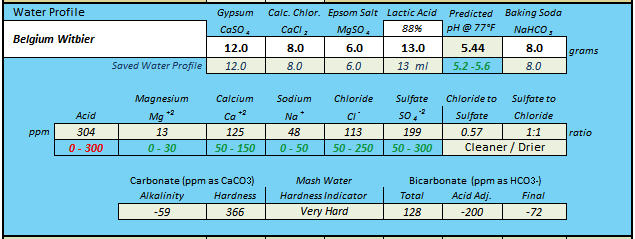
Yes exactly. When brewing a Witbier two years ago I interpreted something Pierre Celis said about his brewing water. When asked what had made Hoegaarden the perfect location for brewing the Witbier style of beer? Pierre Celis replied “Hard water (calcium-rich water) is good for brewing a wheat beer. Also, there were abundant supplies of water in the area. I have a well at my home”.I notice several entries call for lactic acid and sodium bicarbonate. Are you trying to get the sodium up without increasing alkalinity?
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
You can get the sodium up (and also the chloride) simply by adding common sodium chloride salt. No need to combine acids and baking soda to achieve this goal. Unless somehow this acid and base combination imparts flavor benefits via some other means of which I'm totally unaware. Is there such a benefit?
I call what he is doing the "don't care" method. If you want more calcium in your mix but don't want any more sulfate or chloride and don't care about having some lactate or citrate or... get the calcium from calcium carbonate or calcium hydroxide and get it into solution (in the first case) and neutralize it (both cases) with some lactic or citric or ... acid.
The main benefit is limiting the types of mineral additions to just gypsum, calcium chloride, Epsom salt and baking soda. And experimenting with brewing water profiles. Why add alkalinity only to knock it out with acid is a good question. It's the only measurable way to add Na with those four additions.You can get the sodium up (and also the chloride) simply by adding common sodium chloride salt. No need to combine acids and baking soda to achieve this goal. Unless somehow this acid and base combination imparts flavor benefits via some other means of which I'm totally unaware. Is there such a benefit?
I've brewed the same Witbier again as recently as June 2018 using a water profile without baking soda. I was still able to create a very hard water profile ala Pierre Celis but without bicarbonate. According to my notes at 20 minutes into the mash, the actual pH was 5.20 on this batch. Using AJ's voltmeter I soon hope to find out where the 0.24 pH differences are hiding.I call what he is doing the "don't care" method. If you want more calcium in your mix but don't want any more sulfate or chloride and don't care about having some lactate or citrate or... get the calcium from calcium carbonate or calcium hydroxide and get it into solution (in the first case) and neutralize it (both cases) with some lactic or citric or ... acid.
"Mashed @ 122F for 15 minutes raised to 154F over 15 minutes. Then continued mash for 60 minutes. Mash pH @ 20 minutes: 5.20"
Last edited:
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Of late I've come around to the opinion that about 30-60 ppm sodium adds just a little something to the perception of flavor that is lacking in beer without it. I'm amazed at how many brewers who similarly like some level of sodium are abjectly afraid to add NaCl (salt) to their mash and/or sparge water. I've even heard instructions on several occasions that common salt should literally never be added to brewing water. But once the salt molecule fully dissociates into freely migrating Na+ and Cl- ions within water these dissociated ions are absolutely no different from any other Na+ and Cl- ions sourced from elsewhere, such as Cl- from CaCl2 and Na+ from bicarbonate of soda (baking soda). Type for type, an ion is an ion.
But perhaps the oddest thing I've ever heard (more than once) is that good quality "old" water (meaning naturally sourced good tasting water of low to moderate mineralization and alkalinity) is always going to be found superior to "new" water (meaning made from RO or distilled with added minerals). About the only thing of benefit that I can tell is missing from nearly all of such "new" water is a small trace of zinc. But aside from that, most "new" water also typically lacks HCO3- (dissociated bicarbonate ions). This is why I asked above if anyone perceives a certain flavor magic to be associated with the presence of HCO3- ions, even if they are to be wiped out completely by added acid.
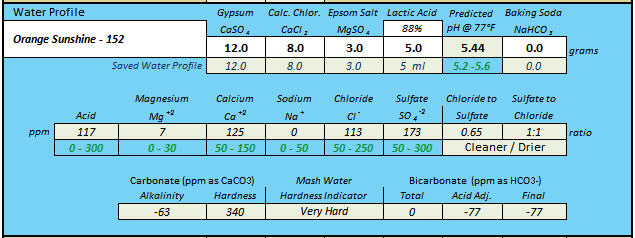
Of all of ScrewyBrewer's water blends which contain both baking soda and lactic acid, if one presumes grams for baking soda and mL's (plus 88% concentration) for lactic acid, only the "CH Evans Brown Ale" water retains any level of HCO3- ions after acidification. Though for the "California Steamin" water it may be a toss-up from one batch to the next as to whether or not any level of free HCO3- remains.
But perhaps the oddest thing I've ever heard (more than once) is that good quality "old" water (meaning naturally sourced good tasting water of low to moderate mineralization and alkalinity) is always going to be found superior to "new" water (meaning made from RO or distilled with added minerals). About the only thing of benefit that I can tell is missing from nearly all of such "new" water is a small trace of zinc. But aside from that, most "new" water also typically lacks HCO3- (dissociated bicarbonate ions). This is why I asked above if anyone perceives a certain flavor magic to be associated with the presence of HCO3- ions, even if they are to be wiped out completely by added acid.
Of all of ScrewyBrewer's water blends which contain both baking soda and lactic acid, if one presumes grams for baking soda and mL's (plus 88% concentration) for lactic acid, only the "CH Evans Brown Ale" water retains any level of HCO3- ions after acidification. Though for the "California Steamin" water it may be a toss-up from one batch to the next as to whether or not any level of free HCO3- remains.
Last edited:

$20.94
$29.99
The Brew Your Own Big Book of Clone Recipes: Featuring 300 Homebrew Recipes from Your Favorite Breweries
Amazon.com

$10.99 ($31.16 / Ounce)
Hornindal Kveik Yeast for Homebrewing - Mead, Cider, Wine, Beer - 10g Packet - Saccharomyces Cerevisiae - Sold by Shadowhive.com
Shadowhive

$719.00
$799.00
EdgeStar KC2000TWIN Full Size Dual Tap Kegerator & Draft Beer Dispenser - Black
Amazon.com

$7.79 ($7.79 / Count)
Craft A Brew - LalBrew Voss™ - Kveik Ale Yeast - For Craft Lagers - Ingredients for Home Brewing - Beer Making Supplies - (1 Pack)
Craft a Brew

$58.16
HUIZHUGS Brewing Equipment Keg Ball Lock Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging Brewing Equipment
xiangshuizhenzhanglingfengshop

$479.00
$559.00
EdgeStar KC1000SS Craft Brew Kegerator for 1/6 Barrel and Cornelius Kegs
Amazon.com

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid Hose Barb)
yunchengshiyanhuqucuichendianzishangwuyouxiangongsi

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid Hose Barb)
Guangshui Weilu You Trading Co., Ltd

$33.99 ($17.00 / Count)
$41.99 ($21.00 / Count)
2 Pack 1 Gallon Large Fermentation Jars with 3 Airlocks and 2 SCREW Lids(100% Airtight Heavy Duty Lid w Silicone) - Wide Mouth Glass Jars w Scale Mark - Pickle Jars for Sauerkraut, Sourdough Starter
Qianfenie Direct

$176.97
1pc Commercial Keg Manifold 2" Tri Clamp,Ball Lock Tapping Head,Pressure Gauge/Adjustable PRV for Kegging,Fermentation Control
hanhanbaihuoxiaoshoudian

$22.00 ($623.23 / Ounce)
AMZLMPKNTW Ball Lock Sample Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging joyful
无为中南商贸有限公司
Of late I've come around to the opinion that about 30-60 ppm sodium adds just a little something to the perception of flavor that is lacking in beer without it.
John Palmer did a couple of trials on sodium perception when AJ and I were helping with the Water book. John reported favorable results and that inspired me to consider it too. Over the years, I've come to the same conclusion. Modest sodium levels are OK, if not good for beer flavor. Don't be afraid to add sodium.
J-T
Active Member
The more I read the more confused I get. Can anyone help me with this?
Hypothesis: you're more confused now than when you first posted.
Correct?
Stand
Well-Known Member
Hypothesis: you're more confused now than when you first posted.
Correct?
Perhaps. Let's see if I can sum up what I've read so far. Tell me how poorly I've gotten this.
1. There is a great degree of disagreement about the basic formulation of pH, and source water profiles seems to complicate things (calculators work better with RO water).
2. Calculating pH is really flipping hard; measuring is better.
3. Baking Soda additions are generally not necessary except to raise the pH although they won't hurt in small amounts(at least for my water).
4. Higher pH than the traditional brewing range is not necessary, but it has some flavor impact that some people enjoy.
5. Moderate levels of sodium are OK, but not necessary (again this is about taste).
6. Y'all know a hell of a lot more than I do.
I updated EZ water calculator to reflect what I understand (or think I understand) thus far:
https://docs.google.com/spreadsheet...e6E-sz3fM4CJ_UN_5COaZLOks/edit#gid=1267915014
As with any formula when eliminating ‘variables’ it makes things more logical and easier to predict.
Predicting mash pH is easier than predicting weather. But the accuracy of both are compared to a measured outcome. Is it raining as predicted? Does mash pH actually match the predicted pH?
I’ll just leave it here. In a Kölsch or a Pilsner style subtle differences are easier to perceive. Brighter color naturally clearer beer better yeast attenuation are more evident. Logically harder to percieve these in a darker or hoppier beer.
Predicting mash pH is easier than predicting weather. But the accuracy of both are compared to a measured outcome. Is it raining as predicted? Does mash pH actually match the predicted pH?
I’ll just leave it here. In a Kölsch or a Pilsner style subtle differences are easier to perceive. Brighter color naturally clearer beer better yeast attenuation are more evident. Logically harder to percieve these in a darker or hoppier beer.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Perhaps. Let's see if I can sum up what I've read so far. Tell me how poorly I've gotten this.
1. There is a great degree of disagreement about the basic formulation of pH, and source water profiles seems to complicate things (calculators work better with RO water).
2. Calculating pH is really flipping hard; measuring is better.
3. Baking Soda additions are generally not necessary except to raise the pH although they won't hurt in small amounts(at least for my water).
4. Higher pH than the traditional brewing range is not necessary, but it has some flavor impact that some people enjoy.
5. Moderate levels of sodium are OK, but not necessary (again this is about taste).
6. Y'all know a hell of a lot more than I do.
1) I feel cheated when a calculator is not capable of utilizing any source water, or water blends. Minerals and alkalinity in source water hardly complicate things (unless the analyticals are not stable over time for your source).
2) What is flipping hard is guessing as to the acidity nature and buffering nature of individual lots of malts/grains when no actual analyticals are present, and only a guesstimate of a Lovibond color is provided.
3) Baking soda raises pH and adds sodium. Its use is dictated by grist needs, water alkalinity, water volume, water mineralization (inherent or added), and desired mash pH. There can be no 'valid' sweeping statement that a little will not hurt.
4) True. But with that said, I'm leaning to the higher end of traditional mash pH's going forward (subject to change if I find that I don't like the general outcome).
5) What can I say, I like a moderate amount of sodium. Many people salt their beer. Ditto their coffee. That's way more than moderate.
6) No way to comment on this one.
Last edited:
Stand
Well-Known Member
I feel bad about giving too little information at the start, but I never expected so many people would be willing to do the work of running the numbers and considering this problem even at the level of detail I started with.
Frankly the response has been overwhelming, and I mean that in the best possible way.
I won't say I understand everything being said here, but I think I have a much better impression of where I need to be.
That said I need to reread this thread a few more times.
Frankly the response has been overwhelming, and I mean that in the best possible way.
I won't say I understand everything being said here, but I think I have a much better impression of where I need to be.
That said I need to reread this thread a few more times.
Don’t feel bad. The amount of information being shared here is unprecedented. And consuming it all is like sipping water from a firehose. Read ask questions and give yourself time to absorb it all.Frankly the response has been overwhelming, and I mean that in the best possible way.
I won't say I understand everything being said here, but I think I have a much better impression of where I need to be.
That said I need to reread this thread a few more times.
It’s all about eliminating variables owning a good quality RO filter and brewing a lot of beer styles. Starting out with pure RO water keeps the main focus on grain pH. And levels the playing field by consistently starting out with the same source water.1) I feel cheated when a calculator is not capable of utilizing any source water, or water blends. Minerals and alkalinity in source water hardly complicate things (unless the analyticals are not stable over time for your source)
The "Quote" feature isn't working today so I'll have to do it manually. I'd say you understand things pretty well.
I'm not sure there is disagreement. Some spreadsheet authors do no understand the basic chemistry, some have eccentric ways of quantifying water data, some ignore essential water information and some, in order to simplify their algorithms make assumptions. Water chemistry is trivially simple until you add bicarbonate (alkalinity). Thus even the ones that are flawed work well with RO water. It is not necessary to match a profile to brew a good beer. The water needs to have only the general characteristics of the water associated with the style to give a good representation of it. One must be somewhat cautious with profiles however as, noted earlier, their authors don't always understand the chemistry. I came across one the other day in which alkalinity was added from sodium bicarbonate only to be taken right back out gain with lime.Perhaps. Let's see if I can sum up what I've read so far. Tell me how poorly I've gotten this.
1. There is a great degree of disagreement about the basic formulation of pH, and source water profiles seems to complicate things (calculators work better with RO water).
The calculations are, actually, amazingly simple. It is getting data that accurately represent the malts actually being used that is hard. A pH measurement will always be best.2. Calculating pH is really flipping hard; measuring is better.
They will always pull mash pH up when in general we are trying to pull it down so, unless you want higher pH for some reason, adding bicarbonate is detrimental. In many cases we find guys acidifying and then adding bicarbonate. This is working against yourself.3. Baking Soda additions are generally not necessary except to raise the pH although they won't hurt in small amounts(at least for my water).
This is really a mater of opinion. Some people firmly believe that stouts should be mashed at higher pH than normal. You will have to determine for yourself whether you are one of those people.4. Higher pH than the traditional brewing range is not necessary, but it has some flavor impact that some people enjoy.
The bit in parentheses is the key.5. Moderate levels of sodium are OK, but not necessary (again this is about taste).
About brewing water chemistry, perhaps but about.....?6. Y'all know a hell of a lot more than I do.
Similar threads
- Replies
- 19
- Views
- 2K
- Replies
- 20
- Views
- 978
- Replies
- 3
- Views
- 479
Latest posts
-
-
-
-
-
-
Spike Conical Heater fails again--second in 3 years--ideas?
- Latest: Indian_villager





![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)








































