We're doing a Smog City "The Nothing Clone" from BYO. Wondering about the water. I'm using my tap water for mash. Wondering if it's good for sparge or if I should be considering distilled. Water chem is something I've struggled with. Thanks.






Thanks for the advice ajdelange. This is my water report. Why sodium bicarbonate instead of chalk just curious? Should I be worried about the sodium?I don't see the water's alkalinity anywhere in the profile but the bicarbonate is listed which suggests that it is around 0.13 mEq/L. At this level you need not worry about treating the water for sparging.
I note that you are proposing to use chalk. This is not a good idea. Use the equivalent amount of sodium bicarbonate and back off on the NaCl if you are worried about the sodium.
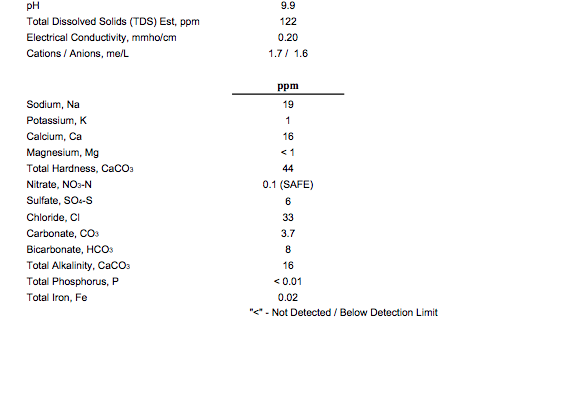
Thanks for the advice ajdelange. This is my water report. Why sodium bicarbonate instead of chalk just curious?
That's really a matter of personal taste. I never add table salt to my beers but as you have added quite a bit I assume you like it. I only mentioned it because you are calling for 1200/100 = 12 mmol of chalk with alkalinity of 1200/50 = 24 mEq. To get that from bicarbonate requires 24 mEq of bicarbonate which will also contribute 24 mmol of sodium which is about half a gram corresponding to the sodium from another 1.4 grams of NaCl.Should I be worried about the sodium?
Enter your email address to join: