Your thoughts on using RO water in LoDo brewing?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
RO with LoDo
- Thread starter Arbe0
- Start date

Help Support Homebrew Talk:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
I don't see it impacting LODO at all. Many LODO brewers build their water from RO.
Looking at LoDo with Gordon Strong recipes that use RO and mostly only using calcium chloride in the mash.
BrewnWKopperKat
ʘ‿ʘ
Looking at LoDo with Gordon Strong recipes that use RO and mostly only using calcium chloride in the mash.
What "LoDo" techniques are you concerned about using ...
... that might be "incompatible"
... with the techniques you are using
... from Strong's books?
Gordon Strong uses RO in his recipes because that's often the common denominator among brewers who design their water. The author cannot anticipate the various well or tap waters out there, so it's more practical to just recommend starting with RO.
The typical LODO functions re mash water include pre-boiling, underletting, mash caps. The oxygen-reducing effects of those functions have nothing to do with whether or not the water is RO, or if one is adding water salts.
There is what's often referred to as the LODO "trifecta"--adding ascorbic acid, sodium or potassium metabisulfite, and Brewtan B to the strike water in an effort to scavenge O2 and limit oxidation in the mash. However, these additions are not impacted by using RO water.
The typical LODO functions re mash water include pre-boiling, underletting, mash caps. The oxygen-reducing effects of those functions have nothing to do with whether or not the water is RO, or if one is adding water salts.
There is what's often referred to as the LODO "trifecta"--adding ascorbic acid, sodium or potassium metabisulfite, and Brewtan B to the strike water in an effort to scavenge O2 and limit oxidation in the mash. However, these additions are not impacted by using RO water.
BrewnWKopperKat
ʘ‿ʘ
.
Last edited:

$20.94
$29.99
The Brew Your Own Big Book of Clone Recipes: Featuring 300 Homebrew Recipes from Your Favorite Breweries
Amazon.com

$33.99 ($17.00 / Count)
$41.99 ($21.00 / Count)
2 Pack 1 Gallon Large Fermentation Jars with 3 Airlocks and 2 SCREW Lids(100% Airtight Heavy Duty Lid w Silicone) - Wide Mouth Glass Jars w Scale Mark - Pickle Jars for Sauerkraut, Sourdough Starter
Qianfenie Direct

$172.35
2 Inch Tri Clamp Keg Manifold With Ball Lock Posts, Pressure Gauge, PRV (0-30 PSI) – Homebrew, Fermentation, Kegging System
wuhanshijiayangzhiyimaoyiyouxiangongsi

$44.99
$49.95
Craft A Brew - Mead Making Kit – Reusable Make Your Own Mead Kit – Yields 1 Gallon of Mead
Craft a Brew

$719.00
$799.00
EdgeStar KC2000TWIN Full Size Dual Tap Kegerator & Draft Beer Dispenser - Black
Amazon.com

$7.79 ($7.79 / Count)
Craft A Brew - LalBrew Voss™ - Kveik Ale Yeast - For Craft Lagers - Ingredients for Home Brewing - Beer Making Supplies - (1 Pack)
Craft a Brew

$49.95 ($0.08 / Fl Oz)
$52.99 ($0.08 / Fl Oz)
Brewer's Best - 1073 - Home Brew Beer Ingredient Kit (5 gallon), (Blueberry Honey Ale) Golden
Amazon.com

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid Hose Barb)
yunchengshiyanhuqucuichendianzishangwuyouxiangongsi

$33.98
DYKWSWYX Heavy Duty Brewing Gloves (1 Pair) - 55CM Long Chemical Resistant Plastic Gloves for Beer & Wine Making, Cleaning, Homebrew Equipment Protection
wuhanshijiayangzhiyimaoyiyouxiangongsi
![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)
$6.95 ($17.38 / Ounce)
$7.47 ($18.68 / Ounce)
Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]
Hobby Homebrew

$76.92 ($2,179.04 / Ounce)
Brewing accessories 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304 Brewing accessories(Gas Hose Barb)
chuhanhandianzishangwu

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Gas MFL)
Guangshui Weilu You Trading Co., Ltd

$479.00
$559.00
EdgeStar KC1000SS Craft Brew Kegerator for 1/6 Barrel and Cornelius Kegs
Amazon.com

$58.16
HUIZHUGS Brewing Equipment Keg Ball Lock Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging Brewing Equipment
xiangshuizhenzhanglingfengshop

$176.97
1pc Commercial Keg Manifold 2" Tri Clamp,Ball Lock Tapping Head,Pressure Gauge/Adjustable PRV for Kegging,Fermentation Control
hanhanbaihuoxiaoshoudian

$22.00 ($623.23 / Ounce)
AMZLMPKNTW Ball Lock Sample Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging joyful
无为中南商贸有限公司
Recipe from Gordon Strongs book "Modern homebrew recipies". I am familiar with LoDo brewing also.What "LoDo" techniques are you concerned about using ...
... that might be "incompatible"
... with the techniques you are using
... from Strong's books?
BrewnWKopperKat
ʘ‿ʘ
.
Last edited:
There should be no problem in using RO with LODO.
However, building a water with only calcium chloride may not produce the best effect in the beer. The 'no sulfate' in German beers or with Noble hops is a thoroughly disproven myth. Better results will include some sulfate in the brewing water.
However, building a water with only calcium chloride may not produce the best effect in the beer. The 'no sulfate' in German beers or with Noble hops is a thoroughly disproven myth. Better results will include some sulfate in the brewing water.
BrewnWKopperKat
ʘ‿ʘ
.
Last edited:
BrewnWKopperKat
ʘ‿ʘ
.
Last edited:
brewbama
Well-Known Member
- Joined
- Sep 20, 2013
- Messages
- 3,802
- Reaction score
- 2,829
+1 to include O2 yeast scavenge as well as pre boil. (It’s more convenient IMO).The typical LODO functions re mash water include pre-boiling, underletting, mash caps. The oxygen-reducing effects of those functions have nothing to do with whether or not the water is RO, or if one is adding water salts.
I agree based on Brewing Better Beer pg 150: “…adding enough calcium for a proper mash and then “seasoning to taste” for the final beer.” (from Tully‘s Session Beers pg 50: “It is common practice to add calcium in some form to the mash to help buffer against rising pH, protect certain malt enzymes from heat, and enhance starch conversion (Sanchez 1999, 43).”)There should be no problem in using RO with LODO.
However, building a water with only calcium chloride may not produce the best effect in the beer. The 'no sulfate' in German beers or with Noble hops is a thoroughly disproven myth. Better results will include some sulfate in the brewing water.
I ‘season to taste’ with CaCl, gypsum, salt, and epsom salt in the kettle because I don’t want anything screwing with my mash pH. Knowing some of the CaCl is consumed in the mash and doesn’t make it to the kettle, I use a 60% reduction of CaCl to calculate kettle additions. (From Tully‘s Session Beers pg 50: “Since only about 40% of the calcium added to the mash carries over to the kettle, a kettle addition may also be warranted depending on the regional water and beer style being created.”)
Last edited:
Sorry, no.Knowing some of the CaCl is consumed in the mash and doesn’t make it to the kettle, I use a 60% reduction of CaCl to calculate kettle additions. (From Tully‘s Session Beers pg 50: “Since only about 40% of the calcium added to the mash carries over to the kettle, a kettle addition may also be warranted depending on the regional water and beer style being created.”)
Calcium is consumed in the mash due to several reactions, but the chloride is not. If you add calcium chloride to the mashing water and raise the chloride content to some value, the resulting wort will have that chloride content. Chloride is very mobile and isn't significantly tied up in the mash.
brewbama
Well-Known Member
- Joined
- Sep 20, 2013
- Messages
- 3,802
- Reaction score
- 2,829
I should have said “60% of Ca“ vs “60% of CaCl”. I agree the chloride is not consumed in the mash. However, the technique described by Talley does work quite well.
For example starting with RO water (using ppm):

So, if I add gypsum to the boil kettle:
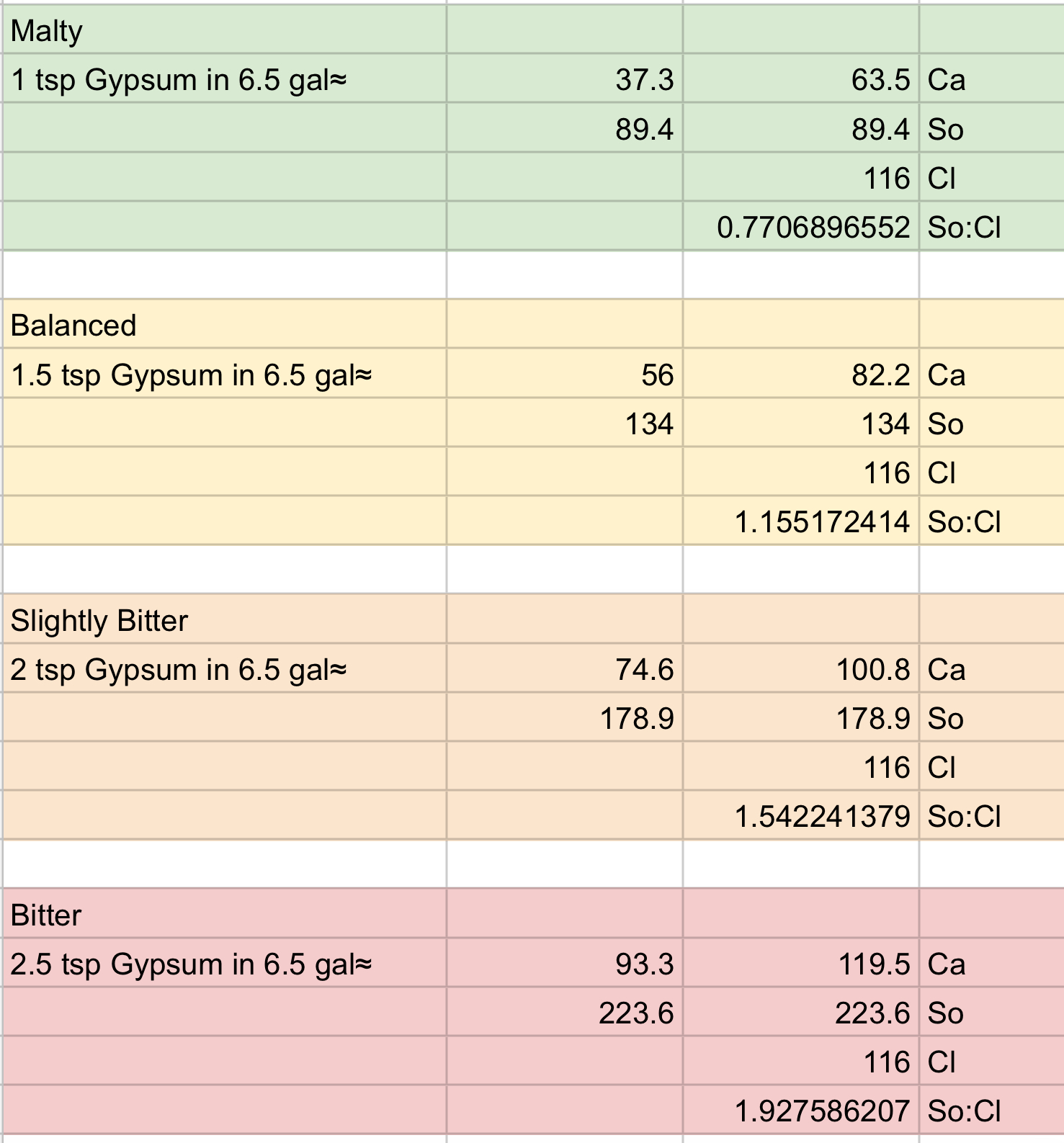
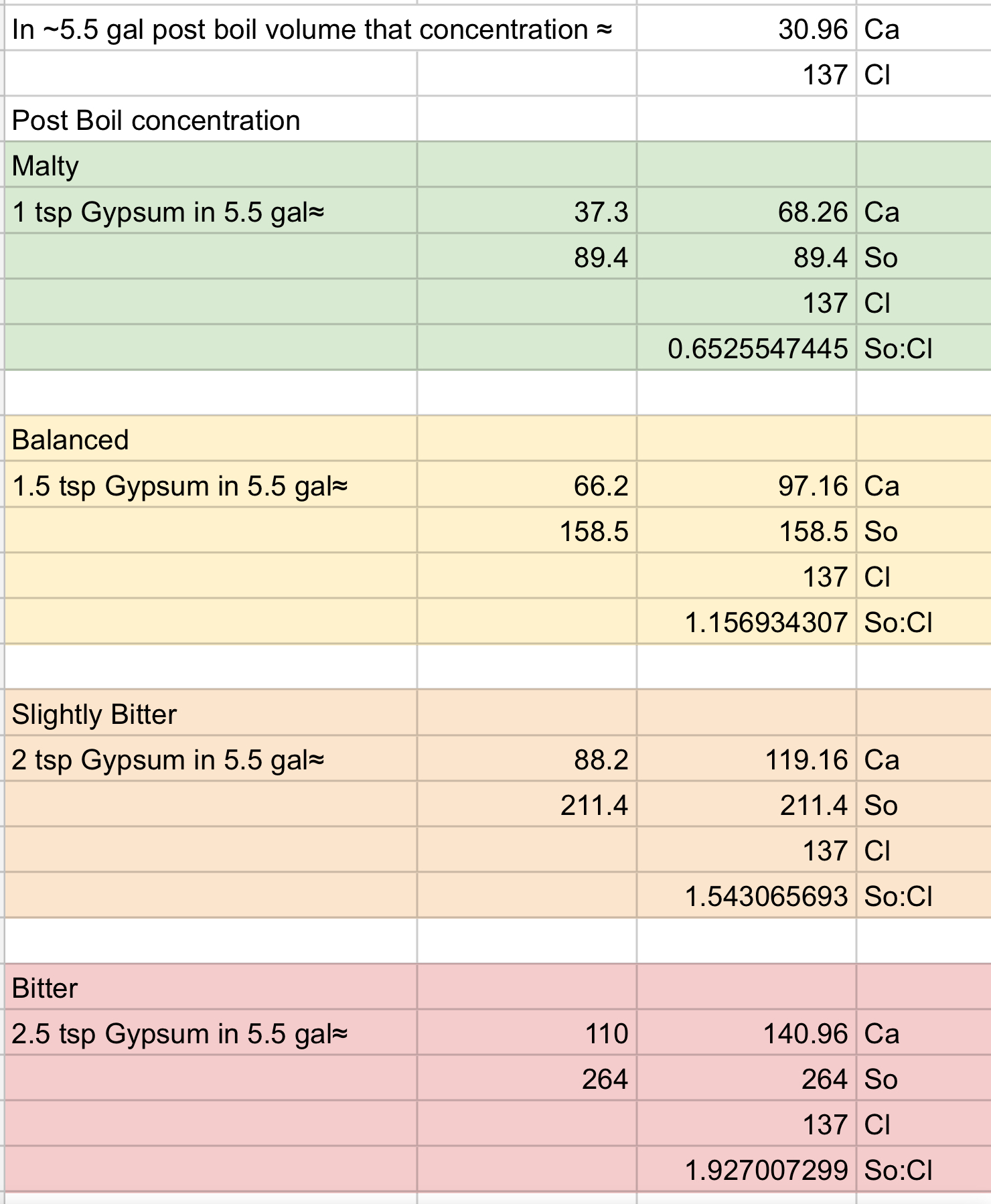
…from what I call ‘Malty’ to ‘Slightly Bitter’ it works quite well within Palmer and Kaminski‘s “typically brewing water has 50-250 ppm of sulfate and 0-250 ppm of chloride” statement. The ‘Bitter’ slightly exceeds their upper limit of sulfate But I’m OK with that on the few occasions I use that profile.
For example starting with RO water (using ppm):

So, if I add gypsum to the boil kettle:


…from what I call ‘Malty’ to ‘Slightly Bitter’ it works quite well within Palmer and Kaminski‘s “typically brewing water has 50-250 ppm of sulfate and 0-250 ppm of chloride” statement. The ‘Bitter’ slightly exceeds their upper limit of sulfate But I’m OK with that on the few occasions I use that profile.
Last edited:
- Joined
- Jun 4, 2022
- Messages
- 21
- Reaction score
- 28
RO water has less DO than tap water. Tap water has 7-9 ppm while RO has 2-3 ppm (could even be less), so if you start with RO then your are ahead in the game.
If you can get the RO water into the boil pot without splashing too much then the boil should be more effective at removing the remaining DO.
Search Youtube for "HSO, LODO & Four Batches" for an interesting video.
(I could not get the link to work)
If you can get the RO water into the boil pot without splashing too much then the boil should be more effective at removing the remaining DO.
Search Youtube for "HSO, LODO & Four Batches" for an interesting video.
(I could not get the link to work)
- Joined
- Jun 4, 2022
- Messages
- 21
- Reaction score
- 28
I bought a DO test kit and currently doing a test on 3 gallons of RO water.
I added 5 grams of bread yeast along with 5 grams of sugar after heating to 100 F.
Results so far:
DO starting point 2.5 ppm
15 minutes 1 ppm
30 minutes 0 ppm
4 hours 0 ppm
24 hours 0 ppm
I'm leaving the pot of water open to the air so the max surface area is exposed. I will continue to check a couple of times a day for the next day or two.
The RO water comes from my under the sink reverse osmosis filter system that I installed for my wife years before I got into brewing.
My tap water test is 4 ppm DO - so the filter system does remove DO.
I plan of doing the test again but using half the yeast.
Just for fun I put some tap water into jar and left plenty of head space. Then shook it for several minutes then tested - results was 9 ppm. From my understanding this is about the max that can be achieved from air.
So far I would would conclude that RO water has lower starting DO and splashing around will increase DO.
I added 5 grams of bread yeast along with 5 grams of sugar after heating to 100 F.
Results so far:
DO starting point 2.5 ppm
15 minutes 1 ppm
30 minutes 0 ppm
4 hours 0 ppm
24 hours 0 ppm
I'm leaving the pot of water open to the air so the max surface area is exposed. I will continue to check a couple of times a day for the next day or two.
The RO water comes from my under the sink reverse osmosis filter system that I installed for my wife years before I got into brewing.
My tap water test is 4 ppm DO - so the filter system does remove DO.
I plan of doing the test again but using half the yeast.
Just for fun I put some tap water into jar and left plenty of head space. Then shook it for several minutes then tested - results was 9 ppm. From my understanding this is about the max that can be achieved from air.
So far I would would conclude that RO water has lower starting DO and splashing around will increase DO.
BrewnWKopperKat
ʘ‿ʘ
Nice!I added 5 grams of bread yeast along with 5 grams of sugar after heating to 100 F.
Results so far:
DO starting point 2.5 ppm
15 minutes 1 ppm
30 minutes 0 ppm
4 hours 0 ppm
24 hours 0 ppm
There are a couple of articles with a number of variations out there in the interwebs. This (link) duckduckgo search should display at least one of them on the 1st page.
- Joined
- Jun 4, 2022
- Messages
- 21
- Reaction score
- 28
I found this link very interesting, I also looked at the wort study they did as well.Nice!
There are a couple of articles with a number of variations out there in the interwebs. This (link) duckduckgo search should display at least one of them on the 1st page.
I was told in the beer making class I took at the LHB store that adding O2 before pitching the yeast was very important.
However, I only use dry yeast (US-05 mostly) and according the the Fermentis website :
"We don’t recommend aerating the wort in normal conditions. The dry yeast has been produced and dried with a specific know-how of the Lesaffre Group, in order to maximize the Ergosterols content of the cells. This allows the yeast to grow/multiply and ferment well.However, you could aerate the wort in particular cases, for example if you recycle the yeast. There is no difference (for the O2) between Ale and Lager."
I don't recycle the yeast so I will no longer be aerating the wort.
Similar threads
- Replies
- 32
- Views
- 2K
Latest posts
-
-
-
For Sale Father's Passing: Homebrew Equip. Mega Sale
- Latest: leedspointbrew
-
-
-
-
-









































